The Effects of Nutrient Signaling Regulators in Combination with Phytocannabinoids on the Senescence-Associated Phenotype in Human Dermal Fibroblasts
- PMID: 35955938
- PMCID: PMC9368899
- DOI: 10.3390/ijms23158804
The Effects of Nutrient Signaling Regulators in Combination with Phytocannabinoids on the Senescence-Associated Phenotype in Human Dermal Fibroblasts
Abstract
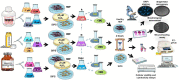
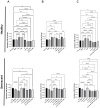
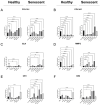
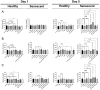
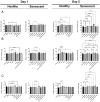
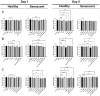
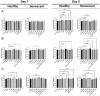
Identifying effective anti-aging compounds is a cornerstone of modern longevity, aging, and skin-health research. There is considerable evidence of the effectiveness of nutrient signaling regulators such as metformin, resveratrol, and rapamycin in longevity and anti-aging studies; however, their potential protective role in skin aging is controversial. In light of the increasing appearance of phytocannabinoids in beauty products without rigorous research on their rejuvenation efficacy, we decided to investigate the potential role of phytocannabinoids in combination with nutrient signaling regulators in skin rejuvenation. Utilizing CCD-1064Sk skin fibroblasts, the effect of metformin, triacetylresveratrol, and rapamycin combined with phytocannabinoids on cellular viability, functional activity, metabolic function, and nuclear architecture was tested. We found triacetylresveratrol combined with cannabidiol increased the viability of skin fibroblasts (p < 0.0001), restored wound-healing functional activity (p < 0.001), reduced metabolic dysfunction, and ameliorated nuclear eccentricity and circularity in senescent fibroblasts (p < 0.01). Conversely, metformin with or without phytocannabinoids did not show any beneficial effects on functional activity, while rapamycin inhibited cell viability (p < 0.01) and the speed of wound healing (p < 0.001). Therefore, triacetylresveratrol and cannabidiol can be a valuable source of biologically active substances used in aging and more studies using animals to confirm the efficacy of cannabidiol combined with triacetylresveratrol should be performed.
Keywords: CBD; THC; aging; fibroblast; metformin; rapamycin; skin; stress-induced premature senescence; triacetylresveratrol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Phytocannabinoids Stimulate Rejuvenation and Prevent Cellular Senescence in Human Dermal Fibroblasts.Cells. 2022 Dec 6;11(23):3939. doi: 10.3390/cells11233939. Cells. 2022. PMID: 36497198 Free PMC article.
-
Modeling of the Senescence-Associated Phenotype in Human Skin Fibroblasts.Int J Mol Sci. 2022 Jun 27;23(13):7124. doi: 10.3390/ijms23137124. Int J Mol Sci. 2022. PMID: 35806127 Free PMC article.
-
Anti-aging pharmacology in cutaneous wound healing: effects of metformin, resveratrol, and rapamycin by local application.Aging Cell. 2017 Oct;16(5):1083-1093. doi: 10.1111/acel.12635. Epub 2017 Jul 5. Aging Cell. 2017. PMID: 28677234 Free PMC article.
-
Shedding Light on the Effects of Calorie Restriction and its Mimetics on Skin Biology.Nutrients. 2020 May 24;12(5):1529. doi: 10.3390/nu12051529. Nutrients. 2020. PMID: 32456324 Free PMC article. Review.
-
From rapalogs to anti-aging formula.Oncotarget. 2017 May 30;8(22):35492-35507. doi: 10.18632/oncotarget.18033. Oncotarget. 2017. PMID: 28548953 Free PMC article. Review.
Cited by
-
Phytocannabinoids Stimulate Rejuvenation and Prevent Cellular Senescence in Human Dermal Fibroblasts.Cells. 2022 Dec 6;11(23):3939. doi: 10.3390/cells11233939. Cells. 2022. PMID: 36497198 Free PMC article.
-
Redox signaling in impaired cascades of wound healing: promising approach.Mol Biol Rep. 2023 Aug;50(8):6927-6936. doi: 10.1007/s11033-023-08589-w. Epub 2023 Jun 21. Mol Biol Rep. 2023. PMID: 37341917 Review.
-
Grp94 Inhibitor HCP1 Inhibits Human Dermal Fibroblast Senescence.Genes (Basel). 2022 Sep 14;13(9):1651. doi: 10.3390/genes13091651. Genes (Basel). 2022. PMID: 36140818 Free PMC article.
-
Cellular rejuvenation: molecular mechanisms and potential therapeutic interventions for diseases.Signal Transduct Target Ther. 2023 Mar 14;8(1):116. doi: 10.1038/s41392-023-01343-5. Signal Transduct Target Ther. 2023. PMID: 36918530 Free PMC article. Review.
-
Cannabinoids and healthy ageing: the potential for extending healthspan and lifespan in preclinical models with an emphasis on Caenorhabditis elegans.Geroscience. 2024 Dec;46(6):5643-5661. doi: 10.1007/s11357-024-01162-8. Epub 2024 May 2. Geroscience. 2024. PMID: 38696056 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

