IKKε Inhibitor Amlexanox Promotes Olaparib Sensitivity through the C/EBP-β-Mediated Transcription of Rad51 in Castrate-Resistant Prostate Cancer
- PMID: 35954347
- PMCID: PMC9367422
- DOI: 10.3390/cancers14153684
IKKε Inhibitor Amlexanox Promotes Olaparib Sensitivity through the C/EBP-β-Mediated Transcription of Rad51 in Castrate-Resistant Prostate Cancer
Abstract
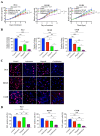
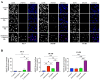
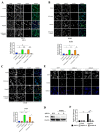
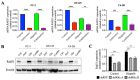
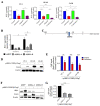
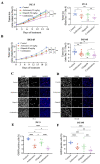
The progression of prostate cancer (PC) is often characterized by the development of castrate-resistant PC (CRPC). Patients with CRPC are treated with a variety of agents including new generation hormonal therapies or chemotherapy. However, as the cancer develops more resistance mechanisms, these drugs eventually become less effective and finding new therapeutic approaches is critical to improving patient outcomes. Previously, we have shown that IKKε depletion and IKKε inhibitors, BX795 and Amlexanox, decrease CRPC cell proliferation in vitro and in vivo and that IKKε inhibitors induce a senescence phenotype accompanied by increased DNA damage and genomic instability in CRPC cells. Here, we describe a new role for IKKε in DNA damage repair involving Rad51 and examine the therapeutic potential of Amlexanox combined with the PARP inhibitor Olaparib in CRPC cell lines. Combining Amlexanox with Olaparib decreased CRPC cell proliferation and enhanced DNA damage through the inhibition of Olaparib-induced Rad51 recruitment and expression in CRPC cells or IKKε-depleted PC-3 cells. We demonstrated that Rad51 promoter activity, measured by luciferase assay, was decreased with Amlexanox treatment or IKKε depletion and that Amlexanox treatment decreased the occupancy of transcription factor C/EBP-β on the Rad51 promoter. Our mouse model also showed that Amlexanox combined with Olaparib inhibited tumor growth of CRPC xenografts. Our study highlights a new role for IKKε in DNA damage repair through the regulation of Rad51 transcription and provides a rationale for the combination of Amlexanox and Olaparib in the treatment of patients with CRPC.
Keywords: DNA damage; DNA damage repair; PARP inhibitors; combination therapy; transcription.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Targeting IKKε in Androgen-Independent Prostate Cancer Causes Phenotypic Senescence and Genomic Instability.Mol Cancer Ther. 2022 Mar 1;21(3):407-418. doi: 10.1158/1535-7163.MCT-21-0519. Mol Cancer Ther. 2022. PMID: 34965959 Free PMC article.
-
IκB-Kinase-epsilon (IKKε) over-expression promotes the growth of prostate cancer through the C/EBP-β dependent activation of IL-6 gene expression.Oncotarget. 2017 Feb 28;8(9):14487-14501. doi: 10.18632/oncotarget.11629. Oncotarget. 2017. PMID: 27577074 Free PMC article.
-
Dual TBK1/IKKε inhibitor amlexanox mitigates palmitic acid-induced hepatotoxicity and lipoapoptosis in vitro.Toxicology. 2020 Nov;444:152579. doi: 10.1016/j.tox.2020.152579. Epub 2020 Sep 6. Toxicology. 2020. PMID: 32905826
-
Olaparib for the treatment of metastatic prostate cancer.Future Oncol. 2021 Jul;17(19):2413-2429. doi: 10.2217/fon-2020-1245. Epub 2021 Mar 26. Future Oncol. 2021. PMID: 33769071 Review.
-
Role of IKKε in the Metabolic Diseases: Physiology, Pathophysiology, and Pharmacology.Front Pharmacol. 2022 May 19;13:888588. doi: 10.3389/fphar.2022.888588. eCollection 2022. Front Pharmacol. 2022. PMID: 35662709 Free PMC article. Review.
Cited by
-
Growth and Migration Blocking Effect of Nanaomycin K, a Compound Produced by Streptomyces sp., on Prostate Cancer Cell Lines In Vitro and In Vivo.Cancers (Basel). 2023 May 10;15(10):2684. doi: 10.3390/cancers15102684. Cancers (Basel). 2023. PMID: 37345021 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

