Therapeutic Targeting Notch2 Protects Bone Micro-Vasculatures from Methotrexate Chemotherapy-Induced Adverse Effects in Rats
- PMID: 35954226
- PMCID: PMC9367713
- DOI: 10.3390/cells11152382
Therapeutic Targeting Notch2 Protects Bone Micro-Vasculatures from Methotrexate Chemotherapy-Induced Adverse Effects in Rats
Abstract
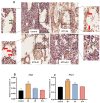
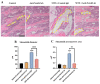
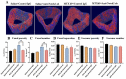
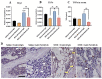
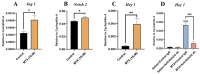
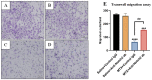
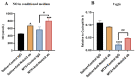
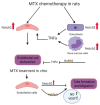
Intensive cancer chemotherapy is well known to cause bone vasculature disfunction and damage, but the mechanism is poorly understood and there is a lack of treatment. Using a rat model of methotrexate (MTX) chemotherapy (five once-daily dosses at 0.75 mg/kg), this study investigated the roles of the Notch2 signalling pathway in MTX chemotherapy-induced bone micro-vasculature impairment. Gene expression, histological and micro-computed tomography (micro-CT) analyses revealed that MTX-induced micro-vasculature dilation and regression is associated with the induction of Notch2 activity in endothelial cells and increased production of inflammatory cytokine tumour necrosis factor alpha (TNFα) from osteoblasts (bone forming cells) and bone marrow cells. Blockade of Notch2 by a neutralising antibody ameliorated MTX adverse effects on bone micro-vasculature, both directly by supressing Notch2 signalling in endothelial cells and indirectly via reducing TNFα production. Furthermore, in vitro studies using rat bone marrow-derived endothelial cell revealed that MTX treatment induces Notch2/Hey1 pathway and negatively affects their ability in migration and tube formation, and Notch2 blockade can partially protect endothelial cell functions from MTX damage.
Keywords: bone vasculature; cancer chemotherapy; methotrexate; notch signalling.
Conflict of interest statement
All authors have no conflict of interest to declare.
Figures

Similar articles
-
Notch2 Blockade Mitigates Methotrexate Chemotherapy-Induced Bone Loss and Marrow Adiposity.Cells. 2022 May 2;11(9):1521. doi: 10.3390/cells11091521. Cells. 2022. PMID: 35563828 Free PMC article.
-
Flavonoid genistein protects bone marrow sinusoidal blood vessels from damage by methotrexate therapy in rats.J Cell Physiol. 2019 Jul;234(7):11276-11286. doi: 10.1002/jcp.27785. Epub 2018 Nov 22. J Cell Physiol. 2019. PMID: 30565680
-
Methotrexate treatment suppresses osteoblastic differentiation by inducing Notch2 signaling and blockade of Notch2 rescues osteogenesis by preserving Wnt/β-catenin signaling.J Orthop Res. 2022 Oct;40(10):2258-2270. doi: 10.1002/jor.25253. Epub 2022 Jan 3. J Orthop Res. 2022. PMID: 34935186
-
Methotrexate toxicity in growing long bones of young rats: a model for studying cancer chemotherapy-induced bone growth defects in children.J Biomed Biotechnol. 2011;2011:903097. doi: 10.1155/2011/903097. Epub 2011 Mar 17. J Biomed Biotechnol. 2011. PMID: 21541196 Free PMC article. Review.
-
Methotrexate osteopathy: five cases and systematic literature review.Osteoporos Int. 2021 Feb;32(2):225-232. doi: 10.1007/s00198-020-05664-x. Epub 2020 Oct 30. Osteoporos Int. 2021. PMID: 33128074 Review.
Cited by
-
CircLZIC regulates ox-LDL-induced HUVEC cell proliferation and apoptosis via Micro-330-5p/NOTCH2 axis in atherosclerosis.Clin Hemorheol Microcirc. 2024;87(1):115-127. doi: 10.3233/CH-232063. Clin Hemorheol Microcirc. 2024. PMID: 38277288 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

