SIAH1-mediated RPS3 ubiquitination contributes to chemosensitivity in epithelial ovarian cancer
- PMID: 35951361
- PMCID: PMC9417229
- DOI: 10.18632/aging.204211
SIAH1-mediated RPS3 ubiquitination contributes to chemosensitivity in epithelial ovarian cancer
Abstract
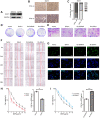
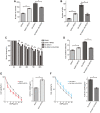
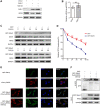
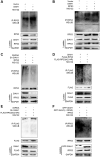
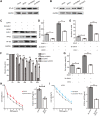
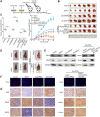
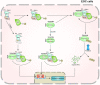
The E3 ligase SIAH1 is deregulated in human cancers and correlated with poor prognosis, but its contributions to chemoresistance in epithelial ovarian cancer (EOC) are not evident. Herein we found that SIAH1 was decreased in EOC tumour tissues and cell lines and negatively correlated with the RPS3 levels. SIAH1 overexpression suppressed tumour cell growth, colony formation, invasion, metastasis, and cisplatin resistance in vivo and in vitro. SIAH1 promoted RPS3 ubiquitination and degradation using the RING-finger domain, and these steps were required for RPS3 localization to the cytoplasm, which led to subsequent NF-κB inactivation and thereby conferred chemosensitivity. Moreover, ectopic expression of RPS3 or depletion of RPS3 ubiquitination mediated by SIAH1 via the K214R mutant significantly impaired cisplatin-induced tumour suppression in cells stably expressing SIAH1. Together, our findings reveal a tumour suppressor function of SIAH1 and provide evidence showing that the SIAH1-RPS3-NF-κB axis may act as an appealing strategy for tackling treatment resistance in EOC.
Keywords: EOC; NF-κB; RPS3; SIAH1; chemoresistance.
Conflict of interest statement
Figures

Similar articles
-
SIAH1 reverses chemoresistance in epithelial ovarian cancer via ubiquitination of YBX-1.Oncogenesis. 2022 Mar 10;11(1):13. doi: 10.1038/s41389-022-00387-6. Oncogenesis. 2022. PMID: 35273154 Free PMC article.
-
SIAH1 Promotes the Pyroptosis of Cardiomyocytes in Diabetic Cardiomyopathy via Regulating IκB-α/NF-κВ Signaling.Crit Rev Eukaryot Gene Expr. 2024;34(5):45-57. doi: 10.1615/CritRevEukaryotGeneExpr.2024052773. Crit Rev Eukaryot Gene Expr. 2024. PMID: 38842203
-
miR-874-3p mitigates cisplatin resistance through modulating NF-κB/inhibitor of apoptosis protein signaling pathway in epithelial ovarian cancer cells.Mol Cell Biochem. 2022 Jan;477(1):307-317. doi: 10.1007/s11010-021-04271-6. Epub 2021 Oct 30. Mol Cell Biochem. 2022. PMID: 34716858
-
Siah1 in cancer and nervous system diseases (Review).Oncol Rep. 2022 Feb;47(2):35. doi: 10.3892/or.2021.8246. Epub 2021 Dec 27. Oncol Rep. 2022. PMID: 34958110 Review.
-
Zooming into the structure-function of RING finger proteins for anti-cancer therapeutic applications.Am J Cancer Res. 2023 Jul 15;13(7):2773-2789. eCollection 2023. Am J Cancer Res. 2023. PMID: 37559981 Free PMC article. Review.
Cited by
-
Protein ubiquitination in ovarian cancer immunotherapy: The progress and therapeutic strategy.Genes Dis. 2023 Oct 29;11(6):101158. doi: 10.1016/j.gendis.2023.101158. eCollection 2024 Nov. Genes Dis. 2023. PMID: 39253578 Free PMC article. Review.
-
Unraveling role of ubiquitination in drug resistance of gynecological cancer.Am J Cancer Res. 2024 May 15;14(5):2523-2537. doi: 10.62347/WYKZ9784. eCollection 2024. Am J Cancer Res. 2024. PMID: 38859858 Free PMC article. Review.
-
SIAH1 ubiquitination-modified HMGCR inhibits lung cancer progression and promotes drug sensitivity through cholesterol synthesis.Cancer Cell Int. 2023 Apr 16;23(1):71. doi: 10.1186/s12935-023-02914-w. Cancer Cell Int. 2023. PMID: 37062828 Free PMC article.
References
-
- Konecny GE, Wang C, Hamidi H, Winterhoff B, Kalli KR, Dering J, Ginther C, Chen HW, Dowdy S, Cliby W, Gostout B, Podratz KC, Keeney G, et al.. Prognostic and therapeutic relevance of molecular subtypes in high-grade serous ovarian cancer. J Natl Cancer Inst. 2014; 106:dju249. 10.1093/jnci/dju249 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

