The Akt Forkhead Box O Transcription Factor Axis Regulates Human Cytomegalovirus Replication
- PMID: 35946797
- PMCID: PMC9426471
- DOI: 10.1128/mbio.01042-22
The Akt Forkhead Box O Transcription Factor Axis Regulates Human Cytomegalovirus Replication
Abstract
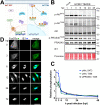
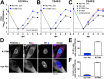
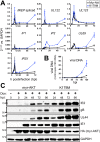
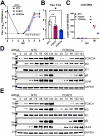
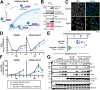
The protein kinase Akt broadly impacts many cellular processes, including mRNA translation, metabolism, apoptosis, and stress responses. Inhibition of phosphatidylinositol 3-kinase (PI3K), a lipid kinase pivotal to Akt activation, triggers various herpesviruses to reactivate from latency. Hence, decreased Akt activity may promote lytic replication. Here, we show that Akt accumulates in an inactive form during human cytomegalovirus (HCMV) infection of permissive fibroblasts, as indicated by hypophosphorylation of sites that activate Akt, decreased phosphorylation of PRAS40, and pronounced nuclear localization of FoxO3a, a substrate that remains cytoplasmic when Akt is active. HCMV strongly activates mTORC1 during lytic infection, suggesting a potential mechanism for Akt inactivation, since mTORC1 negatively regulates PI3K. However, we were surprised to observe that constitutive Akt activity, provided by expression of Akt fused to a myristoylation signal (myr-Akt), caused a 1-log decrease in viral replication, accompanied by defects in viral DNA synthesis and late gene expression. These results indicated that Akt inactivation is required for efficient viral replication, prompting us to address which Akt substrates underpin this requirement. Interestingly, we found that short interfering RNA knockdown of FoxO3a, but not FoxO1, phenocopied the defects caused by myr-Akt, corroborating a role for FoxO3a. Accordingly, a chimeric FoxO3a-estrogen receptor fusion protein, in which nuclear localization is regulated by 4-hydroxytamoxifen instead of Akt, reversed the replication defects caused by myr-Akt. Collectively, our results reveal a role for FoxO transcription factors in HCMV lytic replication and argue that this single class of Akt substrates underpins the requirement for Akt inactivation during productive infection. IMPORTANCE Evidence from diverse herpesvirus infection models suggests that the PI3K/Akt signaling pathway suppresses reactivation from latency and that inactivation of the pathway stimulates viral lytic replication. Here, we show that Akt accumulates in an inactive state during HCMV infection of lytically permissive cells while the presence of constitutive Akt activity causes substantial viral replication defects. Although Akt phosphorylates a diverse array of cellular substrates, we identify an important role for the Forkhead box class O transcription factors. Our findings show that when FoxO3a nuclear localization is decoupled from its negative regulation by Akt, the viral replication defects observed in the presence of constitutively active Akt are reversed. Collectively, our results reveal that HCMV inactivates Akt to promote the nuclear localization of FoxO transcription factors, which strongly implies that FoxOs play critical roles in transactivating cellular and/or viral genes during infection.
Keywords: AKT signaling; cytomegalovirus; herpesviruses; human herpesviruses; metabolism; protein kinases; stress response; transcription factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Helicobacter pylori-associated regulation of forkhead transcription factors FoxO1/3a in human gastric cells.Helicobacter. 2012 Jun;17(3):193-202. doi: 10.1111/j.1523-5378.2012.00939.x. Epub 2012 Mar 20. Helicobacter. 2012. PMID: 22515357 Free PMC article.
-
Phosphorylation of FOXO3a by PI3K/Akt pathway in HK-2 renal proximal tubular epithelial cells exposed to cadmium.Arch Toxicol. 2013 Dec;87(12):2119-27. doi: 10.1007/s00204-013-1077-6. Epub 2013 May 15. Arch Toxicol. 2013. PMID: 23673518
-
FoxO proteins' nuclear retention and BH3-only protein Bim induction evoke mitochondrial dysfunction-mediated apoptosis in berberine-treated HepG2 cells.Free Radic Biol Med. 2014 Nov;76:185-99. doi: 10.1016/j.freeradbiomed.2014.07.039. Epub 2014 Aug 13. Free Radic Biol Med. 2014. PMID: 25128467
-
Introduction to FOXO Biology.Methods Mol Biol. 2019;1890:1-9. doi: 10.1007/978-1-4939-8900-3_1. Methods Mol Biol. 2019. PMID: 30414140 Review.
-
Akt, FoxO and regulation of apoptosis.Biochim Biophys Acta. 2011 Nov;1813(11):1978-86. doi: 10.1016/j.bbamcr.2011.03.010. Epub 2011 Mar 31. Biochim Biophys Acta. 2011. PMID: 21440011 Review.
Cited by
-
Delivery of US28 by incoming HCMV particles rapidly attenuates Akt activity to suppress HCMV lytic replication in monocytes.Sci Signal. 2024 Aug 27;17(851):eadn8727. doi: 10.1126/scisignal.adn8727. Epub 2024 Aug 27. Sci Signal. 2024. PMID: 39190708 Free PMC article.
-
Human cytomegalovirus modulates mTORC1 to redirect mRNA translation within quiescently infected monocytes.J Virol. 2024 Feb 20;98(2):e0188823. doi: 10.1128/jvi.01888-23. Epub 2024 Jan 30. J Virol. 2024. PMID: 38289104 Free PMC article.
-
Pioneer factors in viral infection.Front Immunol. 2023 Oct 9;14:1286617. doi: 10.3389/fimmu.2023.1286617. eCollection 2023. Front Immunol. 2023. PMID: 37876935 Free PMC article. Review.
-
Stabilization of the human cytomegalovirus UL136p33 reactivation determinant overcomes the requirement for UL135 for replication in hematopoietic cells.J Virol. 2023 Aug 31;97(8):e0014823. doi: 10.1128/jvi.00148-23. Epub 2023 Aug 11. J Virol. 2023. PMID: 37565749 Free PMC article.
-
Human cytomegalovirus attenuates AKT activity by destabilizing insulin receptor substrate proteins.bioRxiv [Preprint]. 2023 Apr 17:2023.04.17.537203. doi: 10.1101/2023.04.17.537203. bioRxiv. 2023. Update in: J Virol. 2023 Oct 31;97(10):e0056323. doi: 10.1128/jvi.00563-23 PMID: 37131605 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
