Effect of Intravenous Tirofiban vs Placebo Before Endovascular Thrombectomy on Functional Outcomes in Large Vessel Occlusion Stroke: The RESCUE BT Randomized Clinical Trial
- PMID: 35943471
- PMCID: PMC9364124
- DOI: 10.1001/jama.2022.12584
Effect of Intravenous Tirofiban vs Placebo Before Endovascular Thrombectomy on Functional Outcomes in Large Vessel Occlusion Stroke: The RESCUE BT Randomized Clinical Trial
Abstract
Importance: Tirofiban is a highly selective nonpeptide antagonist of glycoprotein IIb/IIIa receptor, which reversibly inhibits platelet aggregation. It remains uncertain whether intravenous tirofiban is effective to improve functional outcomes for patients with large vessel occlusion ischemic stroke undergoing endovascular thrombectomy.
Objective: To assess the efficacy and adverse events of intravenous tirofiban before endovascular thrombectomy for acute ischemic stroke secondary to large vessel occlusion.
Design, setting, and participants: This investigator-initiated, randomized, double-blind, placebo-controlled trial was implemented at 55 hospitals in China, enrolling 948 patients with stroke and proximal intracranial large vessel occlusion presenting within 24 hours of time last known well. Recruitment took place between October 10, 2018, and October 31, 2021, with final follow-up on January 15, 2022.
Interventions: Participants received intravenous tirofiban (n = 463) or placebo (n = 485) prior to endovascular thrombectomy.
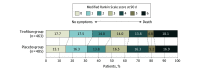
Main outcomes and measures: The primary outcome was disability level at 90 days as measured by overall distribution of the modified Rankin Scale scores from 0 (no symptoms) to 6 (death). The primary safety outcome was the incidence of symptomatic intracranial hemorrhage within 48 hours.
Results: Among 948 patients randomized (mean age, 67 years; 391 [41.2%] women), 948 (100%) completed the trial. The median (IQR) 90-day modified Rankin Scale score in the tirofiban group vs placebo group was 3 (1-4) vs 3 (1-4). The adjusted common odds ratio for a lower level of disability with tirofiban vs placebo was 1.08 (95% CI, 0.86-1.36). Incidence of symptomatic intracranial hemorrhage was 9.7% in the tirofiban group vs 6.4% in the placebo group (difference, 3.3% [95% CI, -0.2% to 6.8%]).
Conclusions and relevance: Among patients with large vessel occlusion acute ischemic stroke undergoing endovascular thrombectomy, treatment with intravenous tirofiban, compared with placebo, before endovascular therapy resulted in no significant difference in disability severity at 90 days. The findings do not support use of intravenous tirofiban before endovascular thrombectomy for acute ischemic stroke.
Trial registration: Chinese Clinical Trial Registry Identifier: ChiCTR-IOR-17014167.
Conflict of interest statement
Figures

Comment in
-
Therapeutic Strategies for Intracranial Atherosclerosis.JAMA. 2022 Aug 9;328(6):529-531. doi: 10.1001/jama.2022.11525. JAMA. 2022. PMID: 35943482 No abstract available.
Similar articles
-
Predictors of outcome in large vessel occlusion stroke patients with intravenous tirofiban treatment: a post hoc analysis of the RESCUE BT clinical trial.BMC Neurol. 2024 Jul 1;24(1):227. doi: 10.1186/s12883-024-03733-w. BMC Neurol. 2024. PMID: 38956505 Free PMC article.
-
Intravenous Tirofiban Versus Alteplase Before Endovascular Treatment in Acute Ischemic Stroke: A Pooled Analysis of the DEVT and RESCUE BT Trials.Stroke. 2024 Apr;55(4):856-865. doi: 10.1161/STROKEAHA.123.044562. Epub 2024 Feb 16. Stroke. 2024. PMID: 38362756
-
Association of Tirofiban With Functional Outcomes After Thrombectomy in Acute Ischemic Stroke Due to Intracranial Atherosclerotic Disease.Neurology. 2023 May 9;100(19):e1996-e2006. doi: 10.1212/WNL.0000000000207194. Epub 2023 Mar 20. Neurology. 2023. PMID: 36941074 Free PMC article. Clinical Trial.
-
Meta-analysis of the efficacy and safety of tirofiban in patients with acute ischaemic stroke undergoing mechanical thrombectomy.Clin Neurol Neurosurg. 2023 May;228:107702. doi: 10.1016/j.clineuro.2023.107702. Epub 2023 Mar 28. Clin Neurol Neurosurg. 2023. PMID: 37058772 Review.
-
Glycoprotein IIb-IIIa inhibitors for acute ischaemic stroke.Cochrane Database Syst Rev. 2014 Mar 8;(3):CD005208. doi: 10.1002/14651858.CD005208.pub3. Cochrane Database Syst Rev. 2014. PMID: 24609741 Review.
Cited by
-
Preoperative and intraoperative tirofiban during endovascular thrombectomy in large vessel occlusion stroke due to large artery atherosclerosis.Eur J Neurol. 2024 Oct;31(10):e16419. doi: 10.1111/ene.16419. Epub 2024 Jul 29. Eur J Neurol. 2024. PMID: 39072930 Free PMC article.
-
Predictors of outcome in large vessel occlusion stroke patients with intravenous tirofiban treatment: a post hoc analysis of the RESCUE BT clinical trial.BMC Neurol. 2024 Jul 1;24(1):227. doi: 10.1186/s12883-024-03733-w. BMC Neurol. 2024. PMID: 38956505 Free PMC article.
-
Association of functional outcomes between intravenous tirofiban and endovascular thrombectomy in imaging-screened patients with large vessel occlusion stroke: a secondary analysis of randomized clinical trial.Int J Surg. 2024 Sep 1;110(9):5505-5517. doi: 10.1097/JS9.0000000000001666. Int J Surg. 2024. PMID: 38788200 Free PMC article. Clinical Trial.
-
The efficacy and safety of continuous intravenous tirofiban for acute ischemic stroke patients treated by endovascular therapy: a meta-analysis.Front Neurol. 2024 Apr 3;15:1286079. doi: 10.3389/fneur.2024.1286079. eCollection 2024. Front Neurol. 2024. PMID: 38633532 Free PMC article.
-
Factors Associated With Premature Termination of Hyperacute Stroke Trials: A Review.J Am Heart Assoc. 2024 Apr 16;13(8):e034115. doi: 10.1161/JAHA.124.034115. Epub 2024 Apr 12. J Am Heart Assoc. 2024. PMID: 38606770 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

