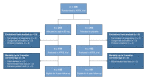
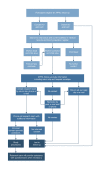
Long-term outcomes following antenatal exposure to low-dose aspirin: study protocol for the 4-year follow-up of the APRIL randomised controlled trial
- PMID: 35940829
- PMCID: PMC9364408
- DOI: 10.1136/bmjopen-2021-060632
Long-term outcomes following antenatal exposure to low-dose aspirin: study protocol for the 4-year follow-up of the APRIL randomised controlled trial
Abstract
Introduction: The use of low-dose aspirin by pregnant women to prevent preterm pre-eclampsia is gradually increasing. The administration of aspirin during pregnancy improves perinatal outcome, which could translate into improved child outcome in the long term. However, antenatal exposure to aspirin could have adverse effects on child development that may manifest later in life. The aim of this follow-up study is to assess the long-term effects of antenatal exposure to low-dose aspirin compared with placebo on survival, (neuro)development, behaviour and general health at 4 years corrected age.
Methods and analysis: This is a follow-up study of the Dutch double-blind randomised controlled APRIL trial which assessed the effectiveness of treatment with aspirin (80 mg daily) compared with placebo for the prevention of preterm birth in women with a previous spontaneous preterm birth. Treatment was initiated before 16 weeks of gestation and continued until 36 weeks or birth. We aim to follow-up all 379 children born to women who participated in the APRIL trial and survived the neonatal period, at the corrected age of 4 years. The main outcomes are (neuro)development as assessed by the Ages and Stages Questionnaire, and behaviour as assessed by the Strength and Difficulties Questionnaire. Additional outcomes include mortality, growth and general health from birth up to 4 years, and a composite outcome including mortality, abnormal (neuro)development and problem behaviour. Analyses will be performed by intention-to-treat using a superiority design.
Ethics and dissemination: Institutional Review Board approval was obtained from the Medical Research Ethics Committee from Amsterdam Medical Center (no. W20 289#20.325). The results will be published in a peer-reviewed journal and presented at conferences.
Trial registration number: The APRIL trial (NTR5675, NL5553; EudraCT number 2015-003220-31) and the APRIL follow-up study (NL8950) are registered in the Dutch trial register. The study is funded by the Amsterdam Reproduction & Development research institute.
Keywords: EPIDEMIOLOGY; Fetal medicine; Maternal medicine; PAEDIATRICS; PERINATOLOGY.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Evaluation of low-dose aspirin in the prevention of recurrent spontaneous preterm labour (the APRIL study): A multicentre, randomised, double-blinded, placebo-controlled trial.PLoS Med. 2022 Feb 1;19(2):e1003892. doi: 10.1371/journal.pmed.1003892. eCollection 2022 Feb. PLoS Med. 2022. PMID: 35104279 Free PMC article. Clinical Trial.
-
Atosiban versus placebo in the treatment of threatened preterm birth between 30 and 34 weeks gestation: study protocol of the 4-year APOSTEL 8 follow-up.BMJ Open. 2024 Jul 18;14(7):e083600. doi: 10.1136/bmjopen-2023-083600. BMJ Open. 2024. PMID: 39025819 Free PMC article.
-
Long-term follow-up of children exposed in-utero to progesterone treatment for prevention of preterm birth: study protocol of the AMPHIA follow-up.BMJ Open. 2021 Sep 21;11(9):e053066. doi: 10.1136/bmjopen-2021-053066. BMJ Open. 2021. PMID: 34548367 Free PMC article.
-
Prenatal interventions for congenital diaphragmatic hernia for improving outcomes.Cochrane Database Syst Rev. 2015 Nov 27;2015(11):CD008925. doi: 10.1002/14651858.CD008925.pub2. Cochrane Database Syst Rev. 2015. PMID: 26611822 Free PMC article. Review.
-
Impact of low-dose aspirin on adverse perinatal outcome: meta-analysis and meta-regression.Ultrasound Obstet Gynecol. 2020 Feb;55(2):157-169. doi: 10.1002/uog.20859. Epub 2020 Jan 8. Ultrasound Obstet Gynecol. 2020. PMID: 31479546 Review.
Cited by
-
Perinatal and obstetric-neonatal outcomes following frozen embryo transfer cycles with a thinner endometrium: a retrospective study.BMC Pregnancy Childbirth. 2024 Nov 12;24(1):741. doi: 10.1186/s12884-024-06946-6. BMC Pregnancy Childbirth. 2024. PMID: 39533220 Free PMC article.
References
-
- National Institute for Health and Care Excellence . Hypertension in pregnancy: diagnosis and management (NICE guideline 133, 2011. Available: https://www.nice.org.uk/guidance/ng133 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous