Semaphorin 4D is upregulated in neurons of diseased brains and triggers astrocyte reactivity
- PMID: 35933420
- PMCID: PMC9356477
- DOI: 10.1186/s12974-022-02509-8
Semaphorin 4D is upregulated in neurons of diseased brains and triggers astrocyte reactivity
Abstract
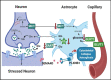
Background: The close interaction and interdependence of astrocytes and neurons allows for the possibility that astrocyte dysfunction contributes to and amplifies neurodegenerative pathology. Molecular pathways that trigger reactive astrocytes may represent important targets to preserve normal homeostatic maintenance and modify disease progression.
Methods: Semaphorin 4D (SEMA4D) expression in the context of disease-associated neuropathology was assessed in postmortem brain sections of patients with Huntington's (HD) and Alzheimer's disease (AD), as well as in mouse models of HD (zQ175) and AD (CVN; APPSwDI/NOS2-/-) by immunohistochemistry. Effects of SEMA4D antibody blockade were assessed in purified astrocyte cultures and in the CVN mouse AD model. CVN mice were treated weekly from 26 to 38 weeks of age; thereafter mice underwent cognitive assessment and brains were collected for histopathology.
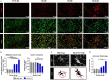
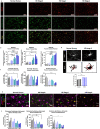
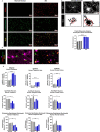
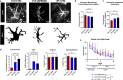
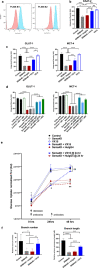
Results: We report here that SEMA4D is upregulated in neurons during progression of neurodegenerative diseases and is a trigger of reactive astrocytes. Evidence of reactive astrocytes in close proximity to neurons expressing SEMA4D is detected in brain sections of patients and mouse models of HD and AD. We further report that SEMA4D-blockade prevents characteristic loss of GABAergic synapses and restores spatial memory and learning in CVN mice, a disease model that appears to reproduce many features of AD-like pathology including neuroinflammation. In vitro mechanistic studies demonstrate that astrocytes express cognate receptors for SEMA4D and that ligand binding triggers morphological variations, and changes in expression of key membrane receptors and enzymes characteristic of reactive astrocytes. These changes include reductions in EAAT-2 glutamate transporter and glutamine synthetase, key enzymes in neurotransmitter recycling, as well as reduced GLUT-1 glucose and MCT-4 lactate transporters, that allow astrocytes to couple energy metabolism with synaptic activity. Antibody blockade of SEMA4D prevented these changes and reversed functional deficits in glucose uptake.
Conclusions: Collectively, these results suggest that SEMA4D blockade may ameliorate disease pathology by preserving normal astrocyte function and reducing the negative consequences of reactive astrogliosis.
Keywords: Alzheimer’s disease; Disease models; Huntington’s disease; Metabolic reprogramming; Neurodegeneration; Neurotransmitter recycling; Pathogenesis; Reactive astrocytes; Semaphorin.
© 2022. The Author(s).
Conflict of interest statement
M. Zauderer, E.E. Evans, T.L. Fisher, V. Mishra, L. Balch, C. Mallow, A. Howell, E. Gersz, C. Reilly and E.S. Smith are employees of Vaccinex, Inc. and own stock and/or stock options in the company.
Figures
Similar articles
-
Anti-Semaphorin 4D Rescues Motor, Cognitive, and Respiratory Phenotypes in a Rett Syndrome Mouse Model.Int J Mol Sci. 2021 Aug 31;22(17):9465. doi: 10.3390/ijms22179465. Int J Mol Sci. 2021. PMID: 34502373 Free PMC article.
-
Anti-semaphorin 4D immunotherapy ameliorates neuropathology and some cognitive impairment in the YAC128 mouse model of Huntington disease.Neurobiol Dis. 2015 Apr;76:46-56. doi: 10.1016/j.nbd.2015.01.002. Epub 2015 Feb 3. Neurobiol Dis. 2015. PMID: 25662335
-
Astrocyte energy and neurotransmitter metabolism in Alzheimer's disease: Integration of the glutamate/GABA-glutamine cycle.Prog Neurobiol. 2022 Oct;217:102331. doi: 10.1016/j.pneurobio.2022.102331. Epub 2022 Jul 21. Prog Neurobiol. 2022. PMID: 35872221 Review.
-
Semaphorin 4D promotes inhibitory synapse formation and suppresses seizures in vivo.Epilepsia. 2018 Jun;59(6):1257-1268. doi: 10.1111/epi.14429. Epub 2018 May 25. Epilepsia. 2018. PMID: 29799628 Free PMC article.
-
Glutamate metabolism and recycling at the excitatory synapse in health and neurodegeneration.Neuropharmacology. 2021 Sep 15;196:108719. doi: 10.1016/j.neuropharm.2021.108719. Epub 2021 Jul 15. Neuropharmacology. 2021. PMID: 34273389 Review.
Cited by
-
Metabotropic glutamate receptors (mGluRs) in epileptogenesis: an update on abnormal mGluRs signaling and its therapeutic implications.Neural Regen Res. 2024 Feb;19(2):360-368. doi: 10.4103/1673-5374.379018. Neural Regen Res. 2024. PMID: 37488891 Free PMC article. Review.
-
Alzheimer's disease and neuroinflammation: will new drugs in clinical trials pave the way to a multi-target therapy?Front Pharmacol. 2023 Jun 2;14:1196413. doi: 10.3389/fphar.2023.1196413. eCollection 2023. Front Pharmacol. 2023. PMID: 37332353 Free PMC article. Review.
-
A case for seeking sex-specific treatments in Alzheimer's disease.Front Aging Neurosci. 2024 Feb 13;16:1346621. doi: 10.3389/fnagi.2024.1346621. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38414633 Free PMC article. Review.
-
Interactions between genes involved in physiological dysregulation and axon guidance: role in Alzheimer's disease.Front Genet. 2023 Aug 31;14:1236509. doi: 10.3389/fgene.2023.1236509. eCollection 2023. Front Genet. 2023. PMID: 37719713 Free PMC article.
-
Navigating the metabolic maze: anomalies in fatty acid and cholesterol processes in Alzheimer's astrocytes.Alzheimers Res Ther. 2024 Mar 23;16(1):63. doi: 10.1186/s13195-024-01430-x. Alzheimers Res Ther. 2024. PMID: 38521950 Free PMC article. Review.
References
-
- Osipovitch M, Asenjo Martinez A, Mariani JN, Cornwell A, Dhaliwal S, Zou L, Chandler-Militello D, Wang S, Li X, Benraiss SJ, et al. Human ESC-derived chimeric mouse models of Huntington's disease reveal cell-intrinsic defects in glial progenitor cell differentiation. Cell Stem Cell. 2019;24(107–122):e107. doi: 10.1016/j.stem.2018.11.010. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

