Emerging mitochondrial signaling mechanisms in cardio-oncology: beyond oxidative stress
- PMID: 35930448
- PMCID: PMC9529263
- DOI: 10.1152/ajpheart.00231.2022
Emerging mitochondrial signaling mechanisms in cardio-oncology: beyond oxidative stress
Abstract
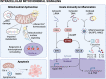
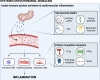
Many anticancer therapies (CTx) have cardiotoxic side effects that limit their therapeutic potential and cause long-term cardiovascular complications in cancer survivors. This has given rise to the field of cardio-oncology, which recognizes the need for basic, translational, and clinical research focused on understanding the complex signaling events that drive CTx-induced cardiovascular toxicity. Several CTx agents cause mitochondrial damage in the form of mitochondrial DNA deletions, mutations, and suppression of respiratory function and ATP production. In this review, we provide a brief overview of the cardiovascular complications of clinically used CTx agents and discuss current knowledge of local and systemic secondary signaling events that arise in response to mitochondrial stress/damage. Mitochondrial oxidative stress has long been recognized as a contributor to CTx-induced cardiotoxicity; thus, we focus on emerging roles for mitochondria in epigenetic regulation, innate immunity, and signaling via noncoding RNAs and mitochondrial hormones. Because data exploring mitochondrial secondary signaling in the context of cardio-oncology are limited, we also draw upon clinical and preclinical studies, which have examined these pathways in other relevant pathologies.
Keywords: DAMPs; cardio-oncology; cardiotoxicity; chemotherapy; mitochondria.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

Similar articles
-
Metabolic Imaging in Cardio-oncology.J Cardiovasc Transl Res. 2020 Jun;13(3):357-366. doi: 10.1007/s12265-019-09927-9. Epub 2019 Nov 6. J Cardiovasc Transl Res. 2020. PMID: 31696405 Review.
-
From Molecular Mechanisms to Clinical Management of Antineoplastic Drug-Induced Cardiovascular Toxicity: A Translational Overview.Antioxid Redox Signal. 2019 Jun 20;30(18):2110-2153. doi: 10.1089/ars.2016.6930. Epub 2017 May 15. Antioxid Redox Signal. 2019. PMID: 28398124 Free PMC article. Review.
-
Cardiotoxicity of Anticancer Drugs: Molecular Mechanisms, Clinical Management and Innovative Treatment.Drug Des Devel Ther. 2024 Sep 12;18:4089-4116. doi: 10.2147/DDDT.S469331. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39286288 Free PMC article. Review.
-
Genetic factors in the pathogenesis of cardio-oncology.J Transl Med. 2024 Aug 5;22(1):739. doi: 10.1186/s12967-024-05537-5. J Transl Med. 2024. PMID: 39103883 Free PMC article. Review.
-
Mitochondrial Dysfunction and Cardiovascular Disease: Pathophysiology and Emerging Therapies.Oxid Med Cell Longev. 2022 Aug 2;2022:9530007. doi: 10.1155/2022/9530007. eCollection 2022. Oxid Med Cell Longev. 2022. Retraction in: Oxid Med Cell Longev. 2024 Jan 9;2024:9785792. doi: 10.1155/2024/9785792 PMID: 35958017 Free PMC article. Retracted. Review.
Cited by
-
Immune interactions in pembrolizumab (PD-1 inhibitor) cancer therapy and cardiovascular complications.Am J Physiol Heart Circ Physiol. 2023 Oct 1;325(4):H751-H767. doi: 10.1152/ajpheart.00378.2023. Epub 2023 Aug 18. Am J Physiol Heart Circ Physiol. 2023. PMID: 37594487 Free PMC article. Review.
-
Cardiovascular aging: spotlight on mitochondria.Am J Physiol Heart Circ Physiol. 2024 Feb 1;326(2):H317-H333. doi: 10.1152/ajpheart.00632.2023. Epub 2023 Dec 1. Am J Physiol Heart Circ Physiol. 2024. PMID: 38038719 Review.
-
Anthracycline chemotherapy, vascular dysfunction and cognitive impairment: burgeoning topics and future directions.Future Cardiol. 2023 Sep;19(11):547-566. doi: 10.2217/fca-2022-0086. Epub 2022 Nov 10. Future Cardiol. 2023. PMID: 36354315 Free PMC article. Review.
-
Historical redlining and breast cancer treatment and survival among older women in the United States.J Natl Cancer Inst. 2023 Jun 8;115(6):652-661. doi: 10.1093/jnci/djad034. J Natl Cancer Inst. 2023. PMID: 36794919 Free PMC article.
-
The impact of greenspace or nature-based interventions on cardiovascular health or cancer-related outcomes: A systematic review of experimental studies.PLoS One. 2022 Nov 23;17(11):e0276517. doi: 10.1371/journal.pone.0276517. eCollection 2022. PLoS One. 2022. PMID: 36417344 Free PMC article.
References
-
- National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Global Health, Committee on Global Health and the Future of the United States. Global Health and the Future Role of the United States. Washington, DC: National Academies Press, 2017. - PubMed
-
- Dempsey N, Rosenthal A, Dabas N, Kropotova Y, Lippman M, Bishopric NH. Trastuzumab-induced cardiotoxicity: a review of clinical risk factors, pharmacologic prevention, and cardiotoxicity of other HER2-directed therapies. Breast Cancer Res Treat 188: 21–36, 2021. doi:10.1007/s10549-021-06280-x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

