Specnuezhenide suppresses diabetes-induced bone loss by inhibiting RANKL-induced osteoclastogenesis
- PMID: 35929595
- PMCID: PMC9827798
- DOI: 10.3724/abbs.2022094
Specnuezhenide suppresses diabetes-induced bone loss by inhibiting RANKL-induced osteoclastogenesis
Abstract
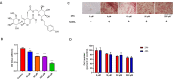
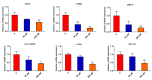
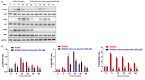
Diabetes osteoporosis is a chronic complication of diabetes mellitus (DM) and is associated with osteoclast formation and enhanced bone resorption. Specnuezhenide (SPN) is an active compound with anti-inflammatory and immunomodulatory properties. However, the roles of SPN in diabetic osteoporosis remain unknown. In this study, primary bone marrow macrophages (BMMs) were pretreated with SPN and were stimulated with receptor activator of nuclear factor kappa B ligand (RANKL; 50 ng/mL) to induce osteoclastogenesis. The number of osteoclasts was detected by tartrate-resistant acid phosphatase (TRAP) staining. The protein levels of cellular oncogene fos/nuclear factor of activated T cells c1 (c-Fos/NFATc1), nuclear factor kappa-B (NF-κB), and mitogen-activated protein kinases (MAPKs) were evaluated by western blot analysis. NF-κB luciferase assays were used to examine the role of SPN in NF-κB activation. The DM model group received a high-glucose, high-fat diet and was then intraperitoneally injected with streptozotocin (STZ). Micro-CT scanning, serum biochemical analysis, histological analysis were used to assess bone loss. We found that SPN suppressed RANKL-induced osteoclast formation and that SPN inhibited the expression of osteoclast-related genes and c-Fos/ NFATc1. SPN inhibited RANKL-induced activation of NF-κB and MAPKs. In vivo experiments revealed that SPN suppressed diabetes-induced bone loss and the number of osteoclasts. Furthermore, SPN decreased the levels of bone turnover markers and increased the levels of runt-related transcription factor 2 (RUNX2), osteoprotegerin (OPG), calcium (Ca) and phosphorus (P). SPN also regulated diabetes-related markers. This study suggests that SPN suppresses diabetes-induced bone loss by inhibiting RANKL-induced osteoclastogenesis, and provides an experimental basis for the treatment of diabetic osteoporosis.
Keywords: MAPK; NF-κB; RANKL; bone loss; osteoclastogenesis; specnuezhenide.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
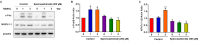

Similar articles
-
Caffeic acid 3,4-dihydroxy-phenethyl ester suppresses receptor activator of NF-κB ligand–induced osteoclastogenesis and prevents ovariectomy-induced bone loss through inhibition of mitogen-activated protein kinase/activator protein 1 and Ca2+–nuclear factor of activated T-cells cytoplasmic 1 signaling pathways.J Bone Miner Res. 2012 Jun;27(6):1298-1308. doi: 10.1002/jbmr.1576. J Bone Miner Res. 2012. PMID: 22337253
-
Trapidil, a platelet-derived growth factor antagonist, inhibits osteoclastogenesis by down-regulating NFATc1 and suppresses bone loss in mice.Biochem Pharmacol. 2013 Sep 15;86(6):782-90. doi: 10.1016/j.bcp.2013.07.015. Epub 2013 Aug 6. Biochem Pharmacol. 2013. PMID: 23928189
-
Acteoside suppresses RANKL-mediated osteoclastogenesis by inhibiting c-Fos induction and NF-κB pathway and attenuating ROS production.PLoS One. 2013 Dec 4;8(12):e80873. doi: 10.1371/journal.pone.0080873. eCollection 2013. PLoS One. 2013. PMID: 24324641 Free PMC article.
-
The Role of Rosavin in the Pathophysiology of Bone Metabolism.Int J Mol Sci. 2024 Feb 9;25(4):2117. doi: 10.3390/ijms25042117. Int J Mol Sci. 2024. PMID: 38396794 Free PMC article. Review.
-
Current Understanding of RANK Signaling in Osteoclast Differentiation and Maturation.Mol Cells. 2017 Oct;40(10):706-713. doi: 10.14348/molcells.2017.0225. Epub 2017 Oct 17. Mol Cells. 2017. PMID: 29047262 Free PMC article. Review.
Cited by
-
The bioelectrical properties of bone tissue.Animal Model Exp Med. 2023 Apr;6(2):120-130. doi: 10.1002/ame2.12300. Epub 2023 Mar 1. Animal Model Exp Med. 2023. PMID: 36856186 Free PMC article. Review.
References
-
- Poiana C, Capatina C. Fracture risk assessment in patients with diabetes mellitus. J Clin Densitometry. . 2017;20:432–443. doi: 10.1016/j.jocd.2017.06.011. - DOI - PubMed
-
- Shu L, Beier E, Sheu T, Zhang H, Zuscik MJ, Puzas EJ, Boyce BF, et al. High-fat diet causes bone loss in young mice by promoting osteoclastogenesis through alteration of the bone marrow environment. Calcif Tissue Int. . 2015;96:313–323. doi: 10.1007/s00223-015-9954-z. - DOI - PMC - PubMed
-
- Stabley JN, Prisby RD, Behnke BJ, Delp MD. Type 2 diabetes alters bone and marrow blood flow and vascular control mechanisms in the ZDF rat. J Endocrinol. . 2015;225:47–58. doi: 10.1530/JOE-14-0514. - DOI - PMC - PubMed
-
- Lecka-Czernik B, Stechschulte LA, Czernik PJ, Dowling AR. High bone mass in adult mice with diet-induced obesity results from a combination of initial increase in bone mass followed by attenuation in bone formation; implications for high bone mass and decreased bone quality in obesity. Mol Cell Endocrinol. . 2015;410:35–41. doi: 10.1016/j.mce.2015.01.001. - DOI - PubMed
-
- Yu W, Zhong L, Yao L, Wei Y, Gui T, Li Z, Kim H, et al. Bone marrow adipogenic lineage precursors promote osteoclastogenesis in bone remodeling and pathologic bone loss. J Clin Investigation. . 2021;131:e140214. doi: 10.1172/JCI140214. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

