Ovarian steroid hormones: A long overlooked but critical contributor to brain aging and Alzheimer's disease
- PMID: 35928995
- PMCID: PMC9344010
- DOI: 10.3389/fnagi.2022.948219
Ovarian steroid hormones: A long overlooked but critical contributor to brain aging and Alzheimer's disease
Abstract
Ovarian hormones, particularly 17β-estradiol, are involved in numerous neurophysiological and neurochemical processes, including those subserving cognitive function. Estradiol plays a key role in the neurobiology of aging, in part due to extensive interconnectivity of the neural and endocrine system. This aspect of aging is fundamental for women's brains as all women experience a drop in circulating estradiol levels in midlife, after menopause. Given the importance of estradiol for brain function, it is not surprising that up to 80% of peri-menopausal and post-menopausal women report neurological symptoms including changes in thermoregulation (vasomotor symptoms), mood, sleep, and cognitive performance. Preclinical evidence for neuroprotective effects of 17β-estradiol also indicate associations between menopause, cognitive aging, and Alzheimer's disease (AD), the most common cause of dementia affecting nearly twice more women than men. Brain imaging studies demonstrated that middle-aged women exhibit increased indicators of AD endophenotype as compared to men of the same age, with onset in perimenopause. Herein, we take a translational approach to illustrate the contribution of ovarian hormones in maintaining cognition in women, with evidence implicating menopause-related declines in 17β-estradiol in cognitive aging and AD risk. We will review research focused on the role of endogenous and exogenous estrogen exposure as a key underlying mechanism to neuropathological aging in women, with a focus on whether brain structure, function and neurochemistry respond to hormone treatment. While still in development, this research area offers a new sex-based perspective on brain aging and risk of AD, while also highlighting an urgent need for better integration between neurology, psychiatry, and women's health practices.
Keywords: Alzheimer’s disease; estrogen; hormone therapy; hormones; menopause; menstrual cycle; neuroimaging; pregnancy.
Copyright © 2022 Jett, Schelbaum, Jang, Boneu Yepez, Dyke, Pahlajani, Diaz Brinton and Mosconi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
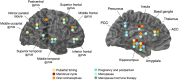
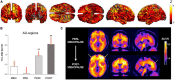
Similar articles
-
Endogenous and Exogenous Estrogen Exposures: How Women's Reproductive Health Can Drive Brain Aging and Inform Alzheimer's Prevention.Front Aging Neurosci. 2022 Mar 9;14:831807. doi: 10.3389/fnagi.2022.831807. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35356299 Free PMC article. Review.
-
Role of Ovarian Hormones in the Modulation of Sleep in Females Across the Adult Lifespan.Endocrinology. 2020 Sep 1;161(9):bqaa128. doi: 10.1210/endocr/bqaa128. Endocrinology. 2020. PMID: 32735650 Free PMC article. Review.
-
Risk for midlife psychosis in women: critical gaps and opportunities in exploring perimenopause and ovarian hormones as mechanisms of risk.Psychol Med. 2022 Jul;52(9):1612-1620. doi: 10.1017/S0033291722001143. Epub 2022 May 18. Psychol Med. 2022. PMID: 35582864 Free PMC article. Review.
-
History of Previous Midlife Estradiol Treatment Permanently Alters Interactions of Brain Insulin-like Growth Factor-1 Signaling and Hippocampal Estrogen Synthesis to Enhance Cognitive Aging in a Rat Model of Menopause.J Neurosci. 2022 Oct 19;42(42):7969-7983. doi: 10.1523/JNEUROSCI.0588-22.2022. Epub 2022 Sep 6. J Neurosci. 2022. PMID: 36261268 Free PMC article.
-
From the 90's to now: A brief historical perspective on more than two decades of estrogen neuroprotection.Brain Res. 2016 Feb 15;1633:96-100. doi: 10.1016/j.brainres.2015.12.044. Epub 2015 Dec 29. Brain Res. 2016. PMID: 26740397 Free PMC article.
Cited by
-
Elevated gonadotropin levels are associated with increased biomarker risk of Alzheimer's disease in midlife women.Front Dement. 2023;2:1303256. doi: 10.3389/frdem.2023.1303256. Epub 2023 Nov 23. Front Dement. 2023. PMID: 38774256 Free PMC article.
-
ERβ mediates sex-specific protection in the App-NL-G-F mouse model of Alzheimer's disease.bioRxiv [Preprint]. 2024 Jul 23:2024.07.22.604543. doi: 10.1101/2024.07.22.604543. bioRxiv. 2024. PMID: 39091856 Free PMC article. Preprint.
-
In vivo brain estrogen receptor density by neuroendocrine aging and relationships with cognition and symptomatology.Sci Rep. 2024 Jun 20;14(1):12680. doi: 10.1038/s41598-024-62820-7. Sci Rep. 2024. PMID: 38902275 Free PMC article.
-
Demand Coupling Drives Neurodegeneration: A Model of Age-Related Cognitive Decline and Dementia.Cells. 2022 Sep 7;11(18):2789. doi: 10.3390/cells11182789. Cells. 2022. PMID: 36139364 Free PMC article. Review.
-
Rationale for a Multi-Factorial Approach for the Reversal of Cognitive Decline in Alzheimer's Disease and MCI: A Review.Int J Mol Sci. 2023 Jan 14;24(2):1659. doi: 10.3390/ijms24021659. Int J Mol Sci. 2023. PMID: 36675177 Free PMC article. Review.
References
-
- Anderson G. L., Chlebowski R. T., Aragaki A. K., Kuller L. H., Manson J. E., Gass M., et al. (2012). Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 13 476–486. 10.1016/S1470-2045(12)70075-X - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

