Cysteine string protein alpha accumulates with early pre-synaptic dysfunction in Alzheimer's disease
- PMID: 35928052
- PMCID: PMC9345313
- DOI: 10.1093/braincomms/fcac192
Cysteine string protein alpha accumulates with early pre-synaptic dysfunction in Alzheimer's disease
Abstract
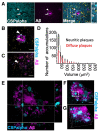
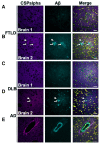
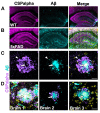
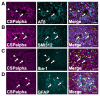
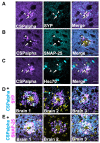
In Alzheimer's disease, synapse loss causes memory and cognitive impairment. However, the mechanisms underlying synaptic degeneration in Alzheimer's disease are not well understood. In the hippocampus, alterations in the level of cysteine string protein alpha, a molecular co-chaperone at the pre-synaptic terminal, occur prior to reductions in synaptophysin, suggesting that it is a very sensitive marker of synapse degeneration in Alzheimer's. Here, we identify putative extracellular accumulations of cysteine string alpha protein, which are proximal to beta-amyloid deposits in post-mortem human Alzheimer's brain and in the brain of a transgenic mouse model of Alzheimer's disease. Cysteine string protein alpha, at least some of which is phosphorylated at serine 10, accumulates near the core of beta-amyloid deposits and does not co-localize with hyperphosphorylated tau, dystrophic neurites or glial cells. Using super-resolution microscopy and array tomography, cysteine string protein alpha was found to accumulate to a greater extent than other pre-synaptic proteins and at a comparatively great distance from the plaque core. This indicates that cysteine string protein alpha is most sensitive to being released from pre-synapses at low concentrations of beta-amyloid oligomers. Cysteine string protein alpha accumulations were also evident in other neurodegenerative diseases, including some fronto-temporal lobar dementias and Lewy body diseases, but only in the presence of amyloid plaques. Our findings are consistent with suggestions that pre-synapses are affected early in Alzheimer's disease, and they demonstrate that cysteine string protein alpha is a more sensitive marker for early pre-synaptic dysfunction than traditional synaptic markers. We suggest that cysteine string protein alpha should be used as a pathological marker for early synaptic disruption caused by beta-amyloid.
Keywords: Alzheimer’s disease; amyloid plaque; cysteine string protein alpha; dystrophy; pre-synapse.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures




Similar articles
-
Evidence that the presynaptic vesicle protein CSPalpha is a key player in synaptic degeneration and protection in Alzheimer's disease.Mol Brain. 2015 Jan 29;8:6. doi: 10.1186/s13041-015-0096-z. Mol Brain. 2015. PMID: 25631211 Free PMC article.
-
Synaptic Mitochondria: An Early Target of Amyloid-β and Tau in Alzheimer's Disease.J Alzheimers Dis. 2021;84(4):1391-1414. doi: 10.3233/JAD-215139. J Alzheimers Dis. 2021. PMID: 34719499 Review.
-
Dissecting phenotypic traits linked to human resilience to Alzheimer's pathology.Brain. 2013 Aug;136(Pt 8):2510-26. doi: 10.1093/brain/awt171. Epub 2013 Jul 3. Brain. 2013. PMID: 23824488 Free PMC article.
-
RAPGEF2 mediates oligomeric Aβ-induced synaptic loss and cognitive dysfunction in the 3xTg-AD mouse model of Alzheimer's disease.Neuropathol Appl Neurobiol. 2021 Aug;47(5):625-639. doi: 10.1111/nan.12686. Epub 2021 Jan 6. Neuropathol Appl Neurobiol. 2021. PMID: 33345400 Free PMC article.
-
Synaptic dysfunction in early phases of Alzheimer's Disease.Handb Clin Neurol. 2022;184:417-438. doi: 10.1016/B978-0-12-819410-2.00022-9. Handb Clin Neurol. 2022. PMID: 35034752 Review.
Cited by
-
On human nanoscale synaptome: Morphology modeling and storage estimation.PLoS One. 2024 Sep 25;19(9):e0310156. doi: 10.1371/journal.pone.0310156. eCollection 2024. PLoS One. 2024. PMID: 39321198 Free PMC article.
-
Decoding transcriptomic signatures of cysteine string protein alpha-mediated synapse maintenance.Proc Natl Acad Sci U S A. 2024 Jun 11;121(24):e2320064121. doi: 10.1073/pnas.2320064121. Epub 2024 Jun 4. Proc Natl Acad Sci U S A. 2024. PMID: 38833477 Free PMC article.
-
Bringing synapses into focus: Recent advances in synaptic imaging and mass-spectrometry for studying synaptopathy.Front Synaptic Neurosci. 2023 Mar 15;15:1130198. doi: 10.3389/fnsyn.2023.1130198. eCollection 2023. Front Synaptic Neurosci. 2023. PMID: 37008679 Free PMC article. Review.
References
-
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: Correlation with cognitive severity. Ann Neurol. 1990;27(5):457–464. - PubMed
-
- Terry RD, Masliah E, Salmon DP, et al. . Physical basis of cognitive alterations in Alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30(4):572–580. - PubMed
-
- Masliah E, Mallory M, Alford M, et al. . Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology. 2001;56(1):127–129. - PubMed
-
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–791. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
