Maturation and substrate processing topography of the Plasmodium falciparum invasion/egress protease plasmepsin X
- PMID: 35927261
- PMCID: PMC9352755
- DOI: 10.1038/s41467-022-32271-7
Maturation and substrate processing topography of the Plasmodium falciparum invasion/egress protease plasmepsin X
Abstract
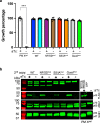
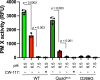
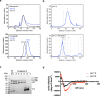
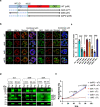
The malaria parasite Plasmodium invades a host erythrocyte, multiplies within a parasitophorous vacuole (PV) and then ruptures the PV and erythrocyte membranes in a process known as egress. Both egress and invasion are controlled by effector proteins discharged from specialized secretory organelles. The aspartic protease plasmepsin X (PM X) regulates activity for many of these effectors, but it is unclear how PM X accesses its diverse substrates that reside in different organelles. PM X also autoprocesses to generate different isoforms. The function of this processing is not understood. We have mapped the self-cleavage sites and have constructed parasites with cleavage site mutations. Surprisingly, a quadruple mutant that remains full-length retains in vitro activity, is trafficked normally, and supports normal egress, invasion and parasite growth. The N-terminal half of the prodomain stays bound to the catalytic domain even after processing and is required for proper intracellular trafficking of PM X. We find that this enzyme cleaves microneme and exoneme substrates before discharge, while the rhoptry substrates that are dependent on PM X activity are cleaved after exoneme discharge into the PV. The data give insight into the temporal, spatial and biochemical control of this unusual but important aspartic protease.
© 2022. The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures


Similar articles
-
Prodomain-driven enzyme dimerization: a pH-dependent autoinhibition mechanism that controls Plasmodium Sub1 activity before merozoite egress.mBio. 2024 Mar 13;15(3):e0019824. doi: 10.1128/mbio.00198-24. Epub 2024 Feb 22. mBio. 2024. PMID: 38386597 Free PMC article.
-
Activation of the Plasmodium Egress Effector Subtilisin-Like Protease 1 Is Mediated by Plasmepsin X Destruction of the Prodomain.mBio. 2023 Apr 25;14(2):e0067323. doi: 10.1128/mbio.00673-23. Epub 2023 Apr 10. mBio. 2023. PMID: 37036362 Free PMC article.
-
The malaria parasite egress protease SUB1 is activated through precise, plasmepsin X-mediated cleavage of the SUB1 prodomain.Biochim Biophys Acta Gen Subj. 2024 Sep;1868(9):130665. doi: 10.1016/j.bbagen.2024.130665. Epub 2024 Jul 3. Biochim Biophys Acta Gen Subj. 2024. PMID: 38969256
-
Targeting malaria parasite proteins to the erythrocyte.Trends Parasitol. 2005 Sep;21(9):399-402. doi: 10.1016/j.pt.2005.07.006. Trends Parasitol. 2005. PMID: 16046185 Review.
-
Vesicle-mediated trafficking of parasite proteins to the host cell cytosol and erythrocyte surface membrane in Plasmodium falciparum infected erythrocytes.Int J Parasitol. 2001 Oct;31(12):1381-91. doi: 10.1016/s0020-7519(01)00256-9. Int J Parasitol. 2001. PMID: 11566305 Review.
Cited by
-
Prodomain-driven enzyme dimerization: a pH-dependent autoinhibition mechanism that controls Plasmodium Sub1 activity before merozoite egress.mBio. 2024 Mar 13;15(3):e0019824. doi: 10.1128/mbio.00198-24. Epub 2024 Feb 22. mBio. 2024. PMID: 38386597 Free PMC article.
-
Profile of Daniel E. Goldberg.Proc Natl Acad Sci U S A. 2023 Sep 26;120(39):e2314435120. doi: 10.1073/pnas.2314435120. Epub 2023 Sep 18. Proc Natl Acad Sci U S A. 2023. PMID: 37722042 Free PMC article. No abstract available.
-
Single-cell transcriptomics reveal transcriptional programs underlying male and female cell fate during Plasmodium falciparum gametocytogenesis.Nat Commun. 2024 Aug 26;15(1):7177. doi: 10.1038/s41467-024-51201-3. Nat Commun. 2024. PMID: 39187486 Free PMC article.
-
Aryl amino acetamides prevent Plasmodium falciparum ring development via targeting the lipid-transfer protein PfSTART1.Nat Commun. 2024 Jun 18;15(1):5219. doi: 10.1038/s41467-024-49491-8. Nat Commun. 2024. PMID: 38890312 Free PMC article.
-
Activation of the Plasmodium Egress Effector Subtilisin-Like Protease 1 Is Mediated by Plasmepsin X Destruction of the Prodomain.mBio. 2023 Apr 25;14(2):e0067323. doi: 10.1128/mbio.00673-23. Epub 2023 Apr 10. mBio. 2023. PMID: 37036362 Free PMC article.
References
-
- WHO. WHO World Malaria Report 2020 (WHO, 2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

