Nanoscale Porphyrin Metal-Organic Frameworks Deliver siRNA for Alleviating Early Pulmonary Fibrosis in Acute Lung Injury
- PMID: 35923570
- PMCID: PMC9339993
- DOI: 10.3389/fbioe.2022.939312
Nanoscale Porphyrin Metal-Organic Frameworks Deliver siRNA for Alleviating Early Pulmonary Fibrosis in Acute Lung Injury
Abstract
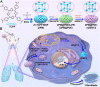
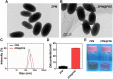
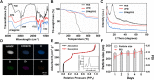
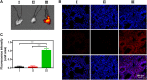
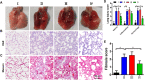
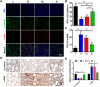
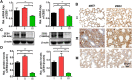
Acute lung injury (ALI) has high mortality and still lacks novel and efficient therapies. Zinc finger E-box binding homeobox 1 and 2 (ZEB1/2) are highly expressed in the early stage of ALI and are positively correlated with the progression of pulmonary fibrosis. Herein, we developed a nanoscale Zr(IV)-based porphyrin metal-organic (ZPM) framework to deliver small interfering ZEB1/2 (siZEB1/2) to alleviate early pulmonary fibrosis during ALI. This pH-responsive nano-ZPM system could effectively protect siRNAs during lung delivery until after internalization and rapidly trigger siRNA release under the mildly acidic environment of the endo/lysosome (pH 4.0-6.5) for transfection and gene silencing. Furthermore, the in vivo studies confirmed that this nano-ZPM system could anchor in inflamed lungs. Moreover, the ZEB1/2 silencing led to increased E-cadherin and decreased α-SMA levels. Overall, the nano-ZPM system was an excellent non-viral vector system to deliver siRNAs to alleviate early pulmonary fibrosis during ALI.
Keywords: endosomal escape; non-viral vector; porphyrin metal-organic framework; pulmonary fibrosis; siRNA.
Copyright © 2022 Weng, Li, Zhang, Duan, Chen, Zhang, Li and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
The fibrotic microenvironment promotes the metastatic seeding of tumor cells into the lungs via mediating the ZEB1-AS1/miR-200b-3p/ZEB1 signaling.Cell Cycle. 2020 Oct;19(20):2701-2719. doi: 10.1080/15384101.2020.1826236. Epub 2020 Oct 5. Cell Cycle. 2020. Retraction in: Cell Cycle. 2022 Dec;21(23):2557. doi: 10.1080/15384101.2022.2097805 PMID: 33017562 Free PMC article. Retracted.
-
[Blockade of programmed death-ligand 1 attenuates indirect acute lung injury in mice through targeting endothelial cells but not epithelial cells].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019 Jan;31(1):37-43. doi: 10.3760/cma.j.issn.2095-4352.2019.01.009. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019. PMID: 30707867 Chinese.
-
Clinical significance of mTOR, ZEB1, ROCK1 expression in lung tissues of pulmonary fibrosis patients.BMC Pulm Med. 2014 Oct 31;14:168. doi: 10.1186/1471-2466-14-168. BMC Pulm Med. 2014. PMID: 25358403 Free PMC article.
-
E-cadherin is transcriptionally activated via suppression of ZEB1 transcriptional repressor by small RNA-mediated gene silencing.PLoS One. 2011;6(12):e28688. doi: 10.1371/journal.pone.0028688. Epub 2011 Dec 21. PLoS One. 2011. PMID: 22205962 Free PMC article.
-
Autophagy Augmentation to Alleviate Immune Response Dysfunction, and Resolve Respiratory and COVID-19 Exacerbations.Cells. 2020 Aug 24;9(9):1952. doi: 10.3390/cells9091952. Cells. 2020. PMID: 32847034 Free PMC article. Review.
Cited by
-
Novel application of nanomedicine for the treatment of acute lung injury: a literature review.Ther Adv Respir Dis. 2024 Jan-Dec;18:17534666241244974. doi: 10.1177/17534666241244974. Ther Adv Respir Dis. 2024. PMID: 38616385 Free PMC article. Review.
-
The sirtuin family in health and disease.Signal Transduct Target Ther. 2022 Dec 29;7(1):402. doi: 10.1038/s41392-022-01257-8. Signal Transduct Target Ther. 2022. PMID: 36581622 Free PMC article. Review.
-
Metal-organic frameworks for food contaminant adsorption and detection.Front Chem. 2023 Jan 18;11:1116524. doi: 10.3389/fchem.2023.1116524. eCollection 2023. Front Chem. 2023. PMID: 36742039 Free PMC article. Review.
References
-
- Aranda-Valderrama P., Kaynar A. M. (2018). The Basic Science and Molecular Mechanisms of Lung Injury and Acute Respiratory Distress Syndrome. Int. Anesthesiol. Clin. Winter 56, 1–25. 10.1097/aia.0000000000000177 PubMed Abstract | 10.1097/aia.0000000000000177 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Beitler J. R., Thompson B. T., Baron R. M., Bastarache J. A., Denlinger L. C., Esserman L., et al. (2022). Advancing Precision Medicine for Acute Respiratory Distress Syndrome. Lancet Respir. Med. 10, 107–120. 10.1016/s2213-2600(21)00157-0 PubMed Abstract | 10.1016/s2213-2600(21)00157-0 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Bian S., Cai H., Cui Y., Liu W., Xiao C. (2021). Nanomedicine-Based Therapeutics to Combat Acute Lung Injury. Ijn Vol. 16, 2247–2269. 10.2147/ijn.s300594 PubMed Abstract | 10.2147/ijn.s300594 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Burnham E. L., Janssen W. J., Riches D. W. H., Moss M., Downey G. P. (2014). The Fibroproliferative Response in Acute Respiratory Distress Syndrome: Mechanisms and Clinical Significance. Eur. Respir. J. 43, 276–285. 10.1183/09031936.00196412 PubMed Abstract | 10.1183/09031936.00196412 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Cao Y., Liu Y., Ping F., Yi L., Zeng Z., Li Y. (2018). miR-200b/c Attenuates Lipopolysaccharide-Induced Early Pulmonary Fibrosis by Targeting ZEB1/2 via P38 MAPK and TGF-Β/smad3 Signaling Pathways. Lab. Invest. 98, 339–359. 10.1038/labinvest.2017.123 PubMed Abstract | 10.1038/labinvest.2017.123 | Google Scholar - DOI - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

