Mast cells promote viral entry of SARS-CoV-2 via formation of chymase/spike protein complex
- PMID: 35921955
- PMCID: PMC9339018
- DOI: 10.1016/j.ejphar.2022.175169
Mast cells promote viral entry of SARS-CoV-2 via formation of chymase/spike protein complex
Abstract
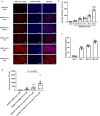
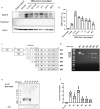
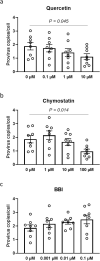
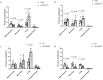
The pulmonary pathological findings associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) result from the release of multiple proinflammatory cytokines, which causes the subsequential damage of the lungs. The current study was undertaken to investigate the responses of mast cells to viral inoculation and their contribution to host defenses from the point of view of viral entry. Pseudovirions, in which the spike glycoprotein of SARS-CoV-2 was incorporated, triggered activation of mast cells, and a mast cell-derived chymase, MCP2, formed a complex with spike protein, which promoted protease-dependent viral entry. According to the quantification results of viral entry, 10 μM quercetin, a mast cell stabilizer, potentially potently inhibited 41.3% of viral entry, while 100 μM chymostatin, which served as a chymase inhibitor, suppressed 52.1% of viral entry, compared to non-treated cells. Study using mast cell-deficient mice showed that the absence of mast cells may influence early viral loading in the upper respiratory tract, which consequently increases the risk of viral invasion into the lower respiratory system. Furthermore, mast cell-deficient mice exhibited ongoing infection in the late phase post-viral inoculation, while clearance of virus-positive cells was observed in wild-type mice. In conclusion, mast cells act as a multifaceted immune modulator that is equipped with both protective effects and pathogenic influences on viral entry of SARS-CoV-2. The utility of mast cell stabilizers and chymase inhibitors in the treatment of SARS-CoV-2-induced acute respiratory syndrome should be optimized regarding the infection stage and the risk of cytokine storm.
Keywords: Chymase; Inhibitor; Mast cell; SARS-CoV-2; Spike.
Copyright © 2022 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Recombinant SARS-CoV-2 Spike Protein Stimulates Secretion of Chymase, Tryptase, and IL-1β from Human Mast Cells, Augmented by IL-33.Int J Mol Sci. 2023 May 30;24(11):9487. doi: 10.3390/ijms24119487. Int J Mol Sci. 2023. PMID: 37298438 Free PMC article.
-
The TMPRSS2 Inhibitor Nafamostat Reduces SARS-CoV-2 Pulmonary Infection in Mouse Models of COVID-19.mBio. 2021 Aug 31;12(4):e0097021. doi: 10.1128/mBio.00970-21. Epub 2021 Aug 3. mBio. 2021. PMID: 34340553 Free PMC article.
-
Optimized Pseudotyping Conditions for the SARS-COV-2 Spike Glycoprotein.J Virol. 2020 Oct 14;94(21):e01062-20. doi: 10.1128/JVI.01062-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32788194 Free PMC article.
-
The expression of hACE2 receptor protein and its involvement in SARS-CoV-2 entry, pathogenesis, and its application as potential therapeutic target.Tumour Biol. 2021;43(1):177-196. doi: 10.3233/TUB-200084. Tumour Biol. 2021. PMID: 34420993 Review.
-
Role of SARS-CoV-2 Spike-Protein-Induced Activation of Microglia and Mast Cells in the Pathogenesis of Neuro-COVID.Cells. 2023 Feb 22;12(5):688. doi: 10.3390/cells12050688. Cells. 2023. PMID: 36899824 Free PMC article. Review.
Cited by
-
Mast Cell Activation Syndrome Update-A Dermatological Perspective.J Pers Med. 2023 Jul 10;13(7):1116. doi: 10.3390/jpm13071116. J Pers Med. 2023. PMID: 37511729 Free PMC article. Review.
-
Mast cell activation in lungs during SARS-CoV-2 infection associated with lung pathology and severe COVID-19.J Clin Invest. 2023 Oct 2;133(19):e149834. doi: 10.1172/JCI149834. J Clin Invest. 2023. PMID: 37561585 Free PMC article.
-
Lentiviral Production Platform.Methods Mol Biol. 2024;2766:163-168. doi: 10.1007/978-1-0716-3682-4_17. Methods Mol Biol. 2024. PMID: 38270876
-
Sambou Bamboo salt™ down-regulates the expression levels of angiotensin-converting enzyme 2 in activated human mast cells.Food Sci Biotechnol. 2023 Oct 24;33(7):1697-1705. doi: 10.1007/s10068-023-01438-3. eCollection 2024 Jun. Food Sci Biotechnol. 2023. PMID: 38623440 Free PMC article.
-
Human Lung Mast Cells as a Possible Reservoir for Coronavirus: A Novel Unrecognized Mechanism for SARS-CoV-2 Immune-Mediated Pathology.Int J Mol Sci. 2024 Jun 13;25(12):6511. doi: 10.3390/ijms25126511. Int J Mol Sci. 2024. PMID: 38928216 Free PMC article.
References
-
- Andersson C.K., Mori M., Bjermer L., Löfdahl C.G., Erjefält J.S. Novel site-specific mast cell subpopulations in the human lung. Thorax. 2009;64:297–305. - PubMed
-
- Caughey G.H., Raymond W.W., Wolters P.J. Angiotensin II generation by mast cell alpha- and beta-chymases. Biochim. Biophys. Acta. 2000;1480:245–257. - PubMed
-
- Conti P., Caraffa A., Tetè G., Gallenga C.E., Ross R., Kritas S.K., Frydas I., Younes A., Di Emidio P., Ronconi G. Mast cells activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19. J Biol Regul Homeost Agents. Copyright 2020 Biolife Sas. 2020:1629–1632. www.biolifesas.org Italy. - PubMed
-
- Dudeck A., Köberle M., Goldmann O., Meyer N., Dudeck J., Lemmens S., Rohde M., Roldán N.G., Dietze-Schwonberg K., Orinska Z., Medina E., Hendrix S., Metz M., Zenclussen A.C., von Stebut E., Biedermann T. Mast cells as protectors of health. J. Allergy Clin. Immunol. 2019;144:S4–s18. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

