Advances in biosynthesis of scopoletin
- PMID: 35918699
- PMCID: PMC9344664
- DOI: 10.1186/s12934-022-01865-7
Advances in biosynthesis of scopoletin
Abstract
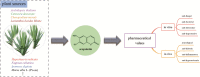
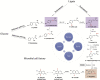
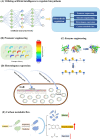
Scopoletin is a typical example of coumarins, which can be produced in plants. Scopoletin acts as a precursor for pharmaceutical and health care products, and also possesses promising biological properties, including antibacterial, anti-tubercular, anti-hypertensive, anti-inflammatory, anti-diabetic, and anti-hyperuricemic activity. Despite the potential benefits, the production of scopoletin using traditional extraction processes from plants is unsatisfactory. In recent years, synthetic biology has developed rapidly and enabled the effective construction of microbial cell factories for production of high value-added chemicals. Herein, this review summarizes the progress of scopoletin biosynthesis in artificial microbial cell factories. The two main pathways of scopoletin biosynthesis are summarized firstly. Then, synthetic microbial cell factories are reviewed as an attractive improvement strategy for biosynthesis. Emerging techniques in synthetic biology and metabolic engineering are introduced as innovative tools for the efficient synthesis of scopoletin. This review showcases the potential of biosynthesis of scopoletin in artificial microbial cell factories.
Keywords: Biosynthesis; Coumarins; Microbial cell factory; Scopoletin; Synthetic biology.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
[Synthetic biology for the synthesis of aromatic natural products: a review].Sheng Wu Gong Cheng Xue Bao. 2021 Jun 25;37(6):2010-2025. doi: 10.13345/j.cjb.210074. Sheng Wu Gong Cheng Xue Bao. 2021. PMID: 34227291 Review. Chinese.
-
Metabolic Engineering of Microbial Cell Factories for Biosynthesis of Flavonoids: A Review.Molecules. 2021 Jul 27;26(15):4522. doi: 10.3390/molecules26154522. Molecules. 2021. PMID: 34361675 Free PMC article. Review.
-
Research progress of engineering microbial cell factories for pigment production.Biotechnol Adv. 2023 Jul-Aug;65:108150. doi: 10.1016/j.biotechadv.2023.108150. Epub 2023 Apr 10. Biotechnol Adv. 2023. PMID: 37044266 Review.
-
Synthetic biology-driven microbial production of folates: Advances and perspectives.Bioresour Technol. 2021 Mar;324:124624. doi: 10.1016/j.biortech.2020.124624. Epub 2020 Dec 28. Bioresour Technol. 2021. PMID: 33434873 Review.
-
Production of ginsenoside compound K by microbial cell factory using synthetic biology-based strategy: a review.Biotechnol Lett. 2023 Feb;45(2):163-174. doi: 10.1007/s10529-022-03326-y. Epub 2022 Dec 22. Biotechnol Lett. 2023. PMID: 36550334 Review.
Cited by
-
Nanomaterial-Based Sensors for Coumarin Detection.ACS Omega. 2024 Jul 5;9(28):30015-30034. doi: 10.1021/acsomega.4c01945. eCollection 2024 Jul 16. ACS Omega. 2024. PMID: 39035881 Free PMC article. Review.
-
Pathway reconstruction and metabolic engineering for the de novo and enhancing production of monacolin J in Pichia pastoris.Bioprocess Biosyst Eng. 2024 Nov;47(11):1789-1801. doi: 10.1007/s00449-024-03069-2. Epub 2024 Jul 31. Bioprocess Biosyst Eng. 2024. PMID: 39085651
-
RNA-Seq and Iso-Seq Reveal the Important Role of COMT and CCoAOMT Genes in Accumulation of Scopoletin in Noni (Morinda citrifolia).Genes (Basel). 2022 Oct 31;13(11):1993. doi: 10.3390/genes13111993. Genes (Basel). 2022. PMID: 36360230 Free PMC article.
-
Uncovering the Mechanism of Scopoletin in Ameliorating Psoriasis-Like Skin Symptoms via Inhibition of PI3K/Akt/mTOR Signaling Pathway.Inflammation. 2024 Nov 22. doi: 10.1007/s10753-024-02188-y. Online ahead of print. Inflammation. 2024. PMID: 39576591
References
-
- Bourgaud F, Hehn A, Larbat R, Doerper S, Gontier E, Kellner S, Matern U. Biosynthesis of coumarins in plants: a major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem Rev. 2006;5:293–308.
-
- Robe K, Izquierdo E, Vignols F, Rouached H, Dubos C. The coumarins: secondary metabolites playing a primary role in plant nutrition and health. Trends Plant Sci. 2021;26:248–259. - PubMed
-
- Kai K, Shimizu B, Mizutani M, Watanabe K, Sakata K. Accumulation of coumarins in Arabidopsis thaliana. Phytochemistry. 2006;67:379–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

