Chronic muscle recordings reveal recovery of forelimb function in spinal injured female rats after cortical epidural stimulation combined with rehabilitation and chondroitinase ABC
- PMID: 35916483
- PMCID: PMC9544922
- DOI: 10.1002/jnr.25111
Chronic muscle recordings reveal recovery of forelimb function in spinal injured female rats after cortical epidural stimulation combined with rehabilitation and chondroitinase ABC
Abstract
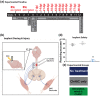
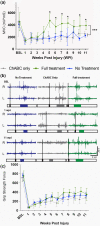
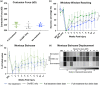
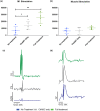
Cervical level spinal cord injury (SCI) can severely impact upper limb muscle function, which is typically assessed in the clinic using electromyography (EMG). Here, we established novel preclinical methodology for EMG assessments of muscle function after SCI in awake freely moving animals. Adult female rats were implanted with EMG recording electrodes in bicep muscles and received bilateral cervical (C7) contusion injuries. Forelimb muscle activity was assessed by recording maximum voluntary contractions during a grip strength task and cortical motor evoked potentials in the biceps. We demonstrate that longitudinal recordings of muscle activity in the same animal are feasible over a chronic post-injury time course and provide a sensitive method for revealing post-injury changes in muscle activity. This methodology was utilized to investigate recovery of muscle function after a novel combination therapy. Cervical contused animals received intraspinal injections of a neuroplasticity-promoting agent (lentiviral-chondroitinase ABC) plus 11 weeks of cortical epidural electrical stimulation (3 h daily, 5 days/week) and behavioral rehabilitation (15 min daily, 5 days/week). Longitudinal monitoring of voluntary and evoked muscle activity revealed significantly increased muscle activity and upper limb dexterity with the combination treatment, compared to a single treatment or no treatment. Retrograde mapping of motor neurons innervating the biceps showed a predominant distribution across spinal segments C5-C8, indicating that treatment effects were likely due to neuroplastic changes in a mixture of intact and injured motor neurons. Thus, longitudinal assessments of muscle function after SCI correlate with skilled reach and grasp performance and reveal functional benefits of a novel combination therapy.
Keywords: EMG; chondroitinase ABC; electrical stimulation; motor evoked potential; physical rehabilitation.
© 2022 The Authors. Journal of Neuroscience Research published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Electrical neuromodulation of the cervical spinal cord facilitates forelimb skilled function recovery in spinal cord injured rats.Exp Neurol. 2017 May;291:141-150. doi: 10.1016/j.expneurol.2017.02.006. Epub 2017 Feb 10. Exp Neurol. 2017. PMID: 28192079 Free PMC article.
-
Chondroitinase gene therapy improves upper limb function following cervical contusion injury.Exp Neurol. 2015 Sep;271:131-5. doi: 10.1016/j.expneurol.2015.05.022. Epub 2015 Jun 1. Exp Neurol. 2015. PMID: 26044197 Free PMC article.
-
Evaluation of optimal electrode configurations for epidural spinal cord stimulation in cervical spinal cord injured rats.J Neurosci Methods. 2015 May 30;247:50-7. doi: 10.1016/j.jneumeth.2015.03.012. Epub 2015 Mar 16. J Neurosci Methods. 2015. PMID: 25791014 Free PMC article.
-
Prolonged acute intermittent hypoxia improves forelimb reach-to-grasp function in a rat model of chronic cervical spinal cord injury.Exp Neurol. 2021 Jun;340:113672. doi: 10.1016/j.expneurol.2021.113672. Epub 2021 Feb 27. Exp Neurol. 2021. PMID: 33652030 Review.
-
Training and anti-CSPG combination therapy for spinal cord injury.Exp Neurol. 2012 May;235(1):26-32. doi: 10.1016/j.expneurol.2011.09.009. Epub 2011 Sep 17. Exp Neurol. 2012. PMID: 21946272 Review.
Cited by
-
Do Pharmacological Treatments Act in Collaboration with Rehabilitation in Spinal Cord Injury Treatment? A Review of Preclinical Studies.Cells. 2024 Feb 27;13(5):412. doi: 10.3390/cells13050412. Cells. 2024. PMID: 38474376 Free PMC article. Review.
References
-
- Adkins‐Muir, D. L. , & Jones, T. A. (2013). Cortical electrical stimulation combined with rehabilitative training: Enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurological Research, 25, 780–788. - PubMed
-
- Ahlborn, P. , Schachner, M. , & Irintchev, A. (2007). One hour electrical stimulation accelerates functional recovery after femoral nerve repair. Experimental Neurology, 208, 137–144. - PubMed
-
- Ahuja, C. S. , Wilson, J. R. , Nori, S. , Kotter, M. R. N. , Druschel, C. , Curt, A. , & Fehlings, M. G. (2017). Traumatic spinal cord injury. Nature Reviews Disease Primers, 3, 17018. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

