Ion channel inhibition with amiodarone or verapamil in symptomatic hospitalized nonintensive-care COVID-19 patients: The ReCOVery-SIRIO randomized trial
- PMID: 35912711
- PMCID: PMC9550324
- DOI: 10.5603/CJ.a2022.0072
Ion channel inhibition with amiodarone or verapamil in symptomatic hospitalized nonintensive-care COVID-19 patients: The ReCOVery-SIRIO randomized trial
Abstract
Background: Ion channel inhibition may offer protection against coronavirus disease 2019 (COVID-19). Inflammation and reduced platelet count occur during COVID-19 but precise quantification of risk thresholds is unclear. The Recov ery-SIRIO study aimed to assess clinical effects of amiodarone and verapamil and to relate patient phenotypes to outcomes.
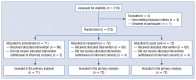
Methods: RECOVERY-SIRIO is a multicenter open-label 1:1:1 investigator-initiated randomized trial with blinded event adjudication. A sample of 804 symptomatic hospitalized nonintensive-care COVID-19 patients, follow-up for 28 days was initially planned.
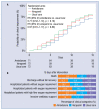
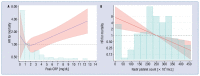
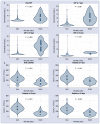
Results: The trial was stopped when a total of 215 patients had been randomized to amiodarone (n = 71), verapamil (n = 72) or standard care alone (n = 72). At 15 days, the hazard ratio (hazard ratio [HR], 95% confidence interval [CI]) for clinical improvement was 0.77 (0.52-1.14) with amiodarone and 0.97 (0.81-1.17) with verapamil as compared to usual care. Clinically relevant associations were found between mortality or lack of clinical improvement and higher peak C-reactive protein (CRP) levels or nadir platelet count at 7, 10 and 15 days. Mortality rate increased by 73% every 5 mg/dL increment in peak CRP (HR 1.73, 95% CI 1.27-2.37) and was two-fold higher for every decrement of 100 units in nadir platelet count (HR 2.19, 95% CI 1.37-3.51). By cluster analysis, thresholds of 5 mg/dL for peak CRP and 187 × 103/mcL for nadir platelet count identified the phenogroup at greatest risk of dying.
Conclusions: In this randomized trial, neither amiodarone nor verapamil were found to significantly accelerate short-term clinical improvement. Peak CRP and nadir platelet counts were associated with increased mortality both in isolation and by cluster analysis.
Keywords: COVID-19; amiodarone; ion-channel inhibition; randomized trial; verapamil.
Conflict of interest statement
Figures
Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Impact of COVID-19 pandemic on acute heart failure admissions and mortality: a multicentre study (COV-HF-SIRIO 6 study).ESC Heart Fail. 2022 Feb;9(1):721-728. doi: 10.1002/ehf2.13680. Epub 2021 Nov 16. ESC Heart Fail. 2022. PMID: 34786869 Free PMC article.
-
IMPACT of PCSK9 inhibition on clinical outcome in patients during the inflammatory stage of the SARS-COV-2 infection: Rationale and protocol of the IMPACT-SIRIO 5 study.Cardiol J. 2022;29(1):140-147. doi: 10.5603/CJ.a2021.0148. Epub 2021 Nov 17. Cardiol J. 2022. PMID: 34787891 Free PMC article. No abstract available.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
Ion channel inhibition against COVID-19: A novel target for clinical investigation.Cardiol J. 2020;27(4):421-424. doi: 10.5603/CJ.a2020.0090. Epub 2020 Jul 9. Cardiol J. 2020. PMID: 32643141 Free PMC article. Review. No abstract available.
Cited by
-
Targeting SARS-CoV-2 and host cell receptor interactions.Antiviral Res. 2023 Feb;210:105514. doi: 10.1016/j.antiviral.2022.105514. Epub 2022 Dec 26. Antiviral Res. 2023. PMID: 36581047 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

