Lung fibroblasts facilitate pre-metastatic niche formation by remodeling the local immune microenvironment
- PMID: 35908547
- PMCID: PMC9830653
- DOI: 10.1016/j.immuni.2022.07.001
Lung fibroblasts facilitate pre-metastatic niche formation by remodeling the local immune microenvironment
Abstract
Primary tumors are drivers of pre-metastatic niche formation, but the coordination by the secondary organ toward metastatic dissemination is underappreciated. Here, by single-cell RNA sequencing and immunofluorescence, we identified a population of cyclooxygenase 2 (COX-2)-expressing adventitial fibroblasts that remodeled the lung immune microenvironment. At steady state, fibroblasts in the lungs produced prostaglandin E2 (PGE2), which drove dysfunctional dendritic cells (DCs) and suppressive monocytes. This lung-intrinsic stromal program was propagated by tumor-associated inflammation, particularly the pro-inflammatory cytokine interleukin-1β, supporting a pre-metastatic niche. Genetic ablation of Ptgs2 (encoding COX-2) in fibroblasts was sufficient to reverse the immune-suppressive phenotypes of lung-resident myeloid cells, resulting in heightened immune activation and diminished lung metastasis in multiple breast cancer models. Moreover, the anti-metastatic activity of DC-based therapy and PD-1 blockade was improved by fibroblast-specific Ptgs2 deletion or dual inhibition of PGE2 receptors EP2 and EP4. Collectively, lung-resident fibroblasts reshape the local immune landscape to facilitate breast cancer metastasis.
Keywords: PGE2; breast cancer; dendritic cells; fibroblasts; immune dysfunction; immunosuppression; immunotherapeutics; lung metastasis; monocytes; pre-metastatic niche.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

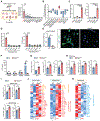
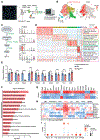

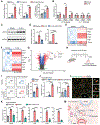
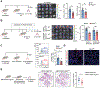
Comment in
-
The lung fibroblast as "soil fertilizer" in breast cancer metastasis.Immunity. 2022 Aug 9;55(8):1336-1339. doi: 10.1016/j.immuni.2022.07.010. Immunity. 2022. PMID: 35947977
Similar articles
-
Upregulation of the S1P3 receptor in metastatic breast cancer cells increases migration and invasion by induction of PGE2 and EP2/EP4 activation.Biochim Biophys Acta. 2016 Nov;1861(11):1840-1851. doi: 10.1016/j.bbalip.2016.09.005. Epub 2016 Sep 9. Biochim Biophys Acta. 2016. PMID: 27616330
-
Cyclooxygenase-2-dependent prostaglandin E2 down-regulates intercellular adhesion molecule-1 expression via EP2/EP4 receptors in interleukin-1beta-stimulated human gingival fibroblasts.J Dent Res. 2000 Dec;79(12):1955-61. doi: 10.1177/00220345000790120601. J Dent Res. 2000. PMID: 11201045
-
Activation of prostaglandin EP4 receptor attenuates the induction of cyclooxygenase-2 expression by EP2 receptor activation in human amnion fibroblasts: implications for parturition.FASEB J. 2019 Jul;33(7):8148-8160. doi: 10.1096/fj.201802642R. Epub 2019 Mar 27. FASEB J. 2019. PMID: 30917001
-
EP4 as a Therapeutic Target for Aggressive Human Breast Cancer.Int J Mol Sci. 2018 Mar 29;19(4):1019. doi: 10.3390/ijms19041019. Int J Mol Sci. 2018. PMID: 29596308 Free PMC article. Review.
-
The lung metastatic niche.J Mol Med (Berl). 2015 Nov;93(11):1185-92. doi: 10.1007/s00109-015-1355-2. J Mol Med (Berl). 2015. PMID: 26489606 Review.
Cited by
-
Key processes in tumor metastasis and therapeutic strategies with nanocarriers: a review.Mol Biol Rep. 2024 Jan 25;51(1):197. doi: 10.1007/s11033-023-08910-7. Mol Biol Rep. 2024. PMID: 38270746 Review.
-
CRISPR-Cas9 Screening Identifies KRAS-Induced COX2 as a Driver of Immunotherapy Resistance in Lung Cancer.Cancer Res. 2024 Jul 15;84(14):2231-2246. doi: 10.1158/0008-5472.CAN-23-2627. Cancer Res. 2024. PMID: 38635884 Free PMC article.
-
Heterogeneity of primary and metastatic CAFs: From differential treatment outcomes to treatment opportunities (Review).Int J Oncol. 2024 May;64(5):54. doi: 10.3892/ijo.2024.5642. Epub 2024 Apr 5. Int J Oncol. 2024. PMID: 38577950 Free PMC article.
-
C-Phycocyanin Prevents Oxidative Stress, Inflammation, and Lung Remodeling in an Ovalbumin-Induced Rat Asthma Model.Int J Mol Sci. 2024 Jun 27;25(13):7031. doi: 10.3390/ijms25137031. Int J Mol Sci. 2024. PMID: 39000141 Free PMC article.
-
S100A10 promotes cancer metastasis via recruitment of MDSCs within the lungs.Oncoimmunology. 2024 Jul 24;13(1):2381803. doi: 10.1080/2162402X.2024.2381803. eCollection 2024. Oncoimmunology. 2024. PMID: 39071160 Free PMC article.
References
-
- Buechler MB, Pradhan RN, Krishnamurty AT, Cox C, Calviello AK, Wang AW, Yang YA, Tam L, Caothien R, Roose-Girma M, et al. (2021). Cross-tissue organization of the fibroblast lineage. Nature 593, 575–579. - PubMed
-
- Coombes RC, Tovey H, Kilburn L, Mansi J, Palmieri C, Bartlett J, Hicks J, Makris A, Evans A, Loibl S, et al. (2021). Effect of Celecoxib vs Placebo as Adjuvant Therapy on Disease-Free Survival Among Patients With Breast Cancer: The REACT Randomized Clinical Trial. JAMA Oncol 7, 1291–1301. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

