First Report of Symmetrical Drug-related Intertriginous and Flexural Exanthema (SDRIFE or Baboon Syndrome) After Erenumab Application for Migraine Prevention
- PMID: 35908264
- PMCID: PMC9633906
- DOI: 10.1007/s40122-022-00417-6
First Report of Symmetrical Drug-related Intertriginous and Flexural Exanthema (SDRIFE or Baboon Syndrome) After Erenumab Application for Migraine Prevention
Abstract
Introduction: Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE), formerly also called baboon syndrome, is characterized by symmetrical erythematous rash with typical localization in the gluteal and intertriginous areas. A type IV delayed hypersensitivity immune response is thought to be responsible for its development. CGRP monoclonal antibodies (CGRP mAbs) are a new class of drugs for the prevention of migraine. We present the first case of SDRIFE occurring in temporal relation to the use of erenumab for migraine prevention.
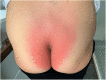
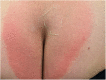
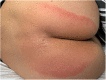
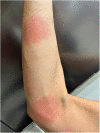
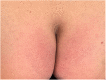
Case: A 48-year-old female patient with migraine received erenumab 140 mg subcutaneously in the thigh area for the prevention of migraine in repetitive cycles, each 1 month apart. Initially, the patient experienced no side effects. After the third cycle, a masseuse incidentally noticed a reddish, circular rash in the buttock area during a back massage. There were no other symptoms. The skin changes resolved spontaneously. Two years later, approximately 40 h after reapplication of erenumab 140 mg, the patient experienced a severe pain in the buttock area centered over the anal crease. The area of pain extended in a circular pattern with approximately 20 cm in diameter. The pain started abruptly and reached a severe intensity within about 30 min. Sitting on the buttocks was no longer possible for the patient. There was marked allodynia and hyperpathia in the entire buttocks region. A flat, broad-based blister-like skin swelling developed in this region. The blisters began opening up on the fourth day after the onset of the skin reaction. In addition, there was a pronounced redness in the entire buttock area. Here, the patient felt a strong burning pain, similar to a scald.
Results: The symptoms lasted for a period of 10 days. From this point on, they fully subsided under concomitant therapy with prednisolone.
Conclusion: SDRIFE as a rare dermatological side effect should be considered in the monitoring of skin lesions during migraine prophylaxis. In view of the high migraine prevalence, knowledge of this uncommon syndrome is important. It is crucial to recognize the relationship between the medication and the circumscribed exanthema occurring distant from the injection site.
Keywords: Baboon syndrome; Dermatological side effect; Erenumab; Exanthema; Migraine; Prevention; SDRIFE; Skin lesions.
© 2022. The Author(s).
Figures
Similar articles
-
Co-occurrence of Symmetrical Drug-Related Intertriginous and Flexural Exanthema (SDRIFE) and Pigmented Fixed Drug Eruption (FDE) in a Single Patient Due to Doxycycline: A Case Report.Indian Dermatol Online J. 2020 Jan 13;11(1):62-64. doi: 10.4103/idoj.IDOJ_104_19. eCollection 2020 Jan-Feb. Indian Dermatol Online J. 2020. PMID: 32055511 Free PMC article.
-
Symmetrical drug-related intertriginous and flexural exanthema/baboon syndrome induced by traditional Chinese medicine.J Cosmet Dermatol. 2022 May;21(5):2200-2204. doi: 10.1111/jocd.14343. Epub 2021 Aug 1. J Cosmet Dermatol. 2022. PMID: 34333850
-
Symmetrical Drug-related Intertriginous and Flexural Exanthema (Baboon Syndrome).Eur J Case Rep Intern Med. 2021 Dec 2;8(12):003029. doi: 10.12890/2021_003029. eCollection 2021. Eur J Case Rep Intern Med. 2021. PMID: 35059336 Free PMC article.
-
Zoledronic acid-associated symmetrical drug-related intertriginous and flexural exanthema (SDRIFE): report of baboon syndrome in a woman with recurrent metastatic breast cancer after receiving zoledronic acid.Dermatol Online J. 2015 Aug 15;21(8):13030/qt5kk0g864. Dermatol Online J. 2015. PMID: 26437156 Review.
-
A systematic approach to systemic contact dermatitis and symmetric drug-related intertriginous and flexural exanthema (SDRIFE): a closer look at these conditions and an approach to intertriginous eruptions.Am J Clin Dermatol. 2011 Jun 1;12(3):171-80. doi: 10.2165/11539080-000000000-00000. Am J Clin Dermatol. 2011. PMID: 21469762 Review.
Cited by
-
Monoclonal Antibodies against Calcitonin Gene-Related Peptide for Migraine Prophylaxis: A Systematic Review of Real-World Data.Cells. 2022 Dec 29;12(1):143. doi: 10.3390/cells12010143. Cells. 2022. PMID: 36611935 Free PMC article. Review.
-
Drug-related side effects and adverse reactions in the treatment of migraine: a bibliometric and visual analysis.Front Neurol. 2024 Feb 6;15:1342111. doi: 10.3389/fneur.2024.1342111. eCollection 2024. Front Neurol. 2024. PMID: 38379705 Free PMC article.
-
Quantitative proteomics reveals CLR interactome in primary human cells.J Biol Chem. 2024 Jun;300(6):107399. doi: 10.1016/j.jbc.2024.107399. Epub 2024 May 20. J Biol Chem. 2024. PMID: 38777147 Free PMC article.
References
-
- De Risi-Pugliese T, Barailler H, Hamelin A, et al. Symmetrical drug-related intertriginous and flexural exanthema: a little-known drug allergy. J Allergy Clin Immunol Pract. 2020;8(3185–3189):e3184. - PubMed
-
- Harbaoui S, Litaiem N. Symmetrical drug-related intertriginous and flexural exanthema. In: StatPearls. Treasure Island; 2022. - PubMed
-
- Neema S, Shaw SC, Gopalakrishnan S. Symmetrical drug-related intertriginous and flexural exanthema. Indian Pediatr. 2020;57:1093. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

