Inhibition of CCL28/CCR10-Mediated eNOS Downregulation Improves Skin Wound Healing in the Obesity-Induced Mouse Model of Type 2 Diabetes
- PMID: 35899992
- PMCID: PMC9501665
- DOI: 10.2337/db21-1108
Inhibition of CCL28/CCR10-Mediated eNOS Downregulation Improves Skin Wound Healing in the Obesity-Induced Mouse Model of Type 2 Diabetes
Abstract
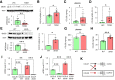
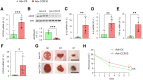
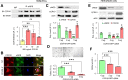
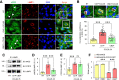
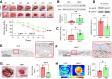
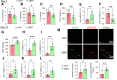
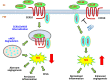
Chronic, nonhealing skin wounds, such as diabetic foot ulcers (DFUs), are common in patients with type 2 diabetes. Here, we investigated the role of chemokine (C-C motif) ligand 28 (CCL28) and its receptor C-C chemokine receptor type 10 (CCR10) in downregulation of endothelial nitric (NO) oxide synthase (eNOS) in association with delayed skin wound healing in the db/db mouse model of type 2 diabetes. We observed reduced eNOS expression and elevated CCL28/CCR10 levels in dorsal skin of db/db mice and subdermal leg biopsy specimens from human subjects with type 2 diabetes. Further interrogation revealed that overexpression of CCR10 reduced eNOS expression, NO bioavailability, and tube formation of human dermal microvascular endothelial cells (HDMVECs) in vitro, which was recapitulated in mouse dorsal skin. In addition, incubation of HDMVECs with CCL28 led to internalization of the CCR10/eNOS complex and colocalization with lysosome-associated membrane protein 1. Finally, topical application of myristoylated CCR10 binding domain 7 amino acid (Myr-CBD7) peptide prevented CCR10-eNOS interaction and subsequent eNOS downregulation, enhanced eNOS/NO levels, eNOS/VEGF-R2+ microvessel density, and blood perfusion, reduced inflammatory cytokine levels, and importantly, decreased wound healing time in db/db mice. Thus, endothelial cell CCR10 activation in genetically obese mice with type 2 diabetes promotes eNOS depletion and endothelial dysfunction, and targeted disruption of CCR10/eNOS interaction improves wound healing.
© 2022 by the American Diabetes Association.
Figures

Similar articles
-
Neutralization of excessive CCL28 improves wound healing in diabetic mice.Front Pharmacol. 2023 Jan 13;14:1087924. doi: 10.3389/fphar.2023.1087924. eCollection 2023. Front Pharmacol. 2023. PMID: 36713846 Free PMC article.
-
CCL28-induced CCR10/eNOS interaction in angiogenesis and skin wound healing.FASEB J. 2020 Apr;34(4):5838-5850. doi: 10.1096/fj.201902060R. Epub 2020 Mar 2. FASEB J. 2020. PMID: 32124475 Free PMC article.
-
Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2).J Biol Chem. 2000 Jul 21;275(29):22313-23. doi: 10.1074/jbc.M001461200. J Biol Chem. 2000. PMID: 10781587
-
Roles of CCR10/CCL27-CCL28 axis in tumour development: mechanisms, diagnostic and therapeutic approaches, and perspectives.Expert Rev Mol Med. 2022 Sep 26;24:e37. doi: 10.1017/erm.2022.28. Expert Rev Mol Med. 2022. PMID: 36155126 Review.
-
CCR10 and its ligands in regulation of epithelial immunity and diseases.Protein Cell. 2012 Aug;3(8):571-80. doi: 10.1007/s13238-012-2927-3. Epub 2012 Jun 8. Protein Cell. 2012. PMID: 22684736 Free PMC article. Review.
Cited by
-
Neutralization of excessive CCL28 improves wound healing in diabetic mice.Front Pharmacol. 2023 Jan 13;14:1087924. doi: 10.3389/fphar.2023.1087924. eCollection 2023. Front Pharmacol. 2023. PMID: 36713846 Free PMC article.
-
Inflammatory protein signatures in individuals with obesity and metabolic syndrome.Sci Rep. 2023 Dec 13;13(1):22185. doi: 10.1038/s41598-023-49643-8. Sci Rep. 2023. PMID: 38092892 Free PMC article.
-
Inhibition of cc chemokine receptor 10 ameliorates osteoarthritis via inhibition of the phosphoinositide-3-kinase/Akt/mammalian target of rapamycin pathway.J Orthop Surg Res. 2024 Mar 1;19(1):158. doi: 10.1186/s13018-024-04642-x. J Orthop Surg Res. 2024. PMID: 38429844 Free PMC article.
-
Racial/ethnic disparities in chronic wounds: Perspectives on linking upstream factors to health outcomes.Wound Repair Regen. 2024 Sep-Oct;32(5):770-779. doi: 10.1111/wrr.13200. Epub 2024 Jun 29. Wound Repair Regen. 2024. PMID: 38943351 Review.
References
-
- Hoffstad O, Mitra N, Walsh J, Margolis DJ. Diabetes, lower-extremity amputation, and death. Diabetes Care 2015;38:1852–1857 - PubMed
-
- Boulton AJ. The diabetic foot: from art to science. The 18th Camillo Golgi lecture. Diabetologia 2004;47:1343–1353 - PubMed
-
- Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med 2016;33:1493–1498 - PubMed
-
- Jhamb S, Vangaveti VN, Malabu UH. Genetic and molecular basis of diabetic foot ulcers: clinical review. J Tissue Viability 2016;25:229–236 - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

