Cebpb Regulates Skeletal Stem Cell Osteogenic Differentiation and Fracture Healing via the WNT/ β-Catenin Pathway
- PMID: 35898655
- PMCID: PMC9314177
- DOI: 10.1155/2022/2091615
Cebpb Regulates Skeletal Stem Cell Osteogenic Differentiation and Fracture Healing via the WNT/ β-Catenin Pathway
Abstract
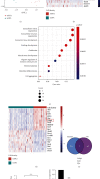
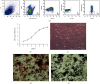
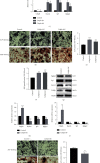
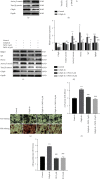
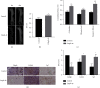
Fracture is the most common traumatic organ injury, and fracture nonunion is a critical clinical challenge. The research on the mechanisms of skeletal stem cell (SSC) differentiation and fracture healing may help develop new treatment strategies and improve the prognosis of patients at high risk of nonunion. Bioinformatic analysis of scRNA-seq data of mouse SSCs and mouse osteoprogenitors was applied to discover major transcription factors for the regulation of SSC differentiation. FACS was used to isolate SSCs prospectively. The expression of Cebpb, osteogenesis-related genes (Runx2, Sp7, and Bglap2), and markers for Notch, Hedgehog, MAPK, BMP2/SMAD, and WNT/β-catenin signaling pathways (Hes1, Gli1, p-Erk1/2, p-Smad1/5/9, and β-catenin) were detected in SSCs with qPCR or western blot, respectively. Alkaline phosphatase assay and alizarin red S staining were used to illustrate the osteogenic differentiation ability of SSCs in vitro. A WNT inhibitor, IWR-1, was further used to explore the mechanism of WNT signaling in the differentiation of SSCs. Micro-CT, mechanical testing, and immunohistochemistry of osteogenic and chondrogenic proteins (Sp7 and Col2α1) were used to demonstrate the capacity of Cebpb knockdown in promoting fracture healing in a monocortical defect model. We found that Cebpb was the crucial transcription factor regulating SSC differentiation. Inhibiting Cebpb in SSCs enhanced the expression of active β-catenin to promote the expression of WNT target genes, thus facilitating the osteogenic differentiation of SSCs. Bone mass, mechanical properties, and osteogenic protein expression were also increased in the Cebpb inhibition group compared to the group without Cebpb inhibition. Collectively, our results proved that Cebpb knockdown promotes SSC osteogenic differentiation and fracture healing via the WNT/β-catenin signaling pathway.
Copyright © 2022 Jiansong Wang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Knockdown of SERPINB2 enhances the osteogenic differentiation of human bone marrow mesenchymal stem cells via activation of the Wnt/β-catenin signalling pathway.Stem Cell Res Ther. 2021 Oct 7;12(1):525. doi: 10.1186/s13287-021-02581-6. Stem Cell Res Ther. 2021. PMID: 34620242 Free PMC article.
-
Berberine promotes bone marrow-derived mesenchymal stem cells osteogenic differentiation via canonical Wnt/β-catenin signaling pathway.Toxicol Lett. 2016 Jan 5;240(1):68-80. doi: 10.1016/j.toxlet.2015.10.007. Epub 2015 Oct 22. Toxicol Lett. 2016. PMID: 26478571
-
[Mechanism of ring finger protein 11 regulating Akt signaling pathway to promote osteogenic differentiation of bone marrow mesenchymal stem cells].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2022 Jan 15;36(1):102-110. doi: 10.7507/1002-1892.202107108. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2022. PMID: 35038807 Free PMC article. Chinese.
-
[RESEARCH PROGRESS OF Hedgehog SIGNALING PATHWAY IN REGULATING BONE FORMATION AND OSTEOGENIC DIFFERENTIATION OF BONE MESENCHYMAL STEM CELLS].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016 Dec 8;30(12):1545-1550. doi: 10.7507/1002-1892.20160318. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016. PMID: 29786349 Review. Chinese.
-
New perspective of skeletal stem cells.Biomater Transl. 2022 Dec 28;3(4):280-294. doi: 10.12336/biomatertransl.2022.04.007. eCollection 2022. Biomater Transl. 2022. PMID: 36846511 Free PMC article. Review.
Cited by
-
A spatiotemporal gene expression and cell atlases of the developing rat ovary.Cell Prolif. 2023 Dec;56(12):e13516. doi: 10.1111/cpr.13516. Epub 2023 Jun 13. Cell Prolif. 2023. PMID: 37309718 Free PMC article.
References
-
- Rodriguez-Buitrago A. F., Mabrouk A., Jahangir A. StatPearls . Publishing LLC; 2021. Tibia nonunion. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

