Cellular and Molecular Mechanism of Pulmonary Fibrosis Post-COVID-19: Focus on Galectin-1, -3, -8, -9
- PMID: 35897786
- PMCID: PMC9332679
- DOI: 10.3390/ijms23158210
Cellular and Molecular Mechanism of Pulmonary Fibrosis Post-COVID-19: Focus on Galectin-1, -3, -8, -9
Abstract
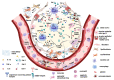
Pulmonary fibrosis is a consequence of the pathological accumulation of extracellular matrix (ECM), which finally leads to lung scarring. Although the pulmonary fibrogenesis is almost known, the last two years of the COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its post effects added new particularities which need to be explored. Many questions remain about how pulmonary fibrotic changes occur within the lungs of COVID-19 patients, and whether the changes will persist long term or are capable of resolving. This review brings together existing knowledge on both COVID-19 and pulmonary fibrosis, starting with the main key players in promoting pulmonary fibrosis, such as alveolar and endothelial cells, fibroblasts, lipofibroblasts, and macrophages. Further, we provide an overview of the main molecular mechanisms driving the fibrotic process in connection with Galactin-1, -3, -8, and -9, together with the currently approved and newly proposed clinical therapeutic solutions given for the treatment of fibrosis, based on their inhibition. The work underlines the particular pathways and processes that may be implicated in pulmonary fibrosis pathogenesis post-SARS-CoV-2 viral infection. The recent data suggest that galectin-1, -3, -8, and -9 could become valuable biomarkers for the diagnosis and prognosis of lung fibrosis post-COVID-19 and promising molecular targets for the development of new and original therapeutic tools to treat the disease.
Keywords: COVID-19; galectin; myofibroblasts; pulmonary fibrosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
COVID-19 and pulmonary fibrosis: A potential role for lung epithelial cells and fibroblasts.Immunol Rev. 2021 Jul;302(1):228-240. doi: 10.1111/imr.12977. Epub 2021 May 24. Immunol Rev. 2021. PMID: 34028807 Free PMC article. Review.
-
Pulmonary fibrosis from molecular mechanisms to therapeutic interventions: lessons from post-COVID-19 patients.Biochem Pharmacol. 2021 Nov;193:114812. doi: 10.1016/j.bcp.2021.114812. Epub 2021 Oct 21. Biochem Pharmacol. 2021. PMID: 34687672 Free PMC article. Review.
-
Potential mechanisms for lung fibrosis associated with COVID-19 infection.QJM. 2023 Jul 28;116(7):487-492. doi: 10.1093/qjmed/hcac206. QJM. 2023. PMID: 36018274 Free PMC article. Review.
-
Pulmonary fibrosis in COVID-19: mechanisms, consequences and targets.QJM. 2023 Oct 6;116(9):750-754. doi: 10.1093/qjmed/hcad092. QJM. 2023. PMID: 37191984 Review.
-
Human-Based Advanced in vitro Approaches to Investigate Lung Fibrosis and Pulmonary Effects of COVID-19.Front Med (Lausanne). 2021 May 7;8:644678. doi: 10.3389/fmed.2021.644678. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34026781 Free PMC article. Review.
Cited by
-
Effect of Home-Based Pulmonary Rehabilitation on Pulmonary Fibrosis.Multidiscip Respir Med. 2024 Jun 5;19(1):950. doi: 10.5826/mrm.2024.950. Multidiscip Respir Med. 2024. PMID: 38836339 Free PMC article.
-
The Role of Cytokines and Molecular Pathways in Lung Fibrosis Following SARS-CoV-2 Infection: A Physiopathologic (Re)view.Biomedicines. 2024 Mar 13;12(3):639. doi: 10.3390/biomedicines12030639. Biomedicines. 2024. PMID: 38540252 Free PMC article. Review.
-
The COVID-19 inflammation and high mortality mechanism trigger.Immunogenetics. 2024 Feb;76(1):15-25. doi: 10.1007/s00251-023-01326-4. Epub 2023 Dec 8. Immunogenetics. 2024. PMID: 38063879 Review.
-
Molecular mechanisms of COVID-19-induced pulmonary fibrosis and epithelial-mesenchymal transition.Front Pharmacol. 2023 Aug 3;14:1218059. doi: 10.3389/fphar.2023.1218059. eCollection 2023. Front Pharmacol. 2023. PMID: 37601070 Free PMC article. Review.
-
Pulmonary fibrosis: A short- or long-term sequelae of severe COVID-19?Chin Med J Pulm Crit Care Med. 2023 Jun;1(2):77-83. doi: 10.1016/j.pccm.2022.12.002. Epub 2023 Jan 23. Chin Med J Pulm Crit Care Med. 2023. PMID: 37388822 Free PMC article. Review.
References
-
- Jafarzadeh A., Chauhan P., Saha B., Jafarzadeh S., Nemati M. Contribution of Monocytes and Macrophages to the Local Tissue Inflammation and Cytokine Storm in COVID-19: Lessons from SARS and MERS, and Potential Therapeutic Interventions. Life Sci. 2020;257:118102. doi: 10.1016/j.lfs.2020.118102. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

