DNA Damage Response Regulation by Histone Ubiquitination
- PMID: 35897775
- PMCID: PMC9332593
- DOI: 10.3390/ijms23158187
DNA Damage Response Regulation by Histone Ubiquitination
Abstract
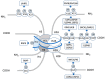
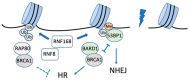
Cells are constantly exposed to numerous genotoxic stresses that induce DNA damage. DNA double-strand breaks (DSBs) are among the most serious damages and should be systematically repaired to preserve genomic integrity. The efficiency of repair is closely associated with chromatin structure, which is regulated by posttranslational modifications of histones, including ubiquitination. Recent evidence shows crosstalk between histone ubiquitination and DNA damage responses, suggesting an integrated model for the systematic regulation of DNA repair. There are two major pathways for DSB repair, viz., nonhomologous end joining and homologous recombination, and the choice of the pathway is partially controlled by posttranslational modifications of histones, including ubiquitination. Histone ubiquitination changes chromatin structure in the vicinity of DSBs and serves as a platform to select and recruit repair proteins; the removal of these modifications by deubiquitinating enzymes suppresses the recruitment of repair proteins and promotes the convergence of repair reactions. This article provides a comprehensive overview of the DNA damage response regulated by histone ubiquitination in response to DSBs.
Keywords: DNA repair; chromatin; histone modifications; ubiquitination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Regulation of Histone Ubiquitination in Response to DNA Double Strand Breaks.Cells. 2020 Jul 16;9(7):1699. doi: 10.3390/cells9071699. Cells. 2020. PMID: 32708614 Free PMC article. Review.
-
Histone ubiquitination in the DNA damage response.DNA Repair (Amst). 2017 Aug;56:92-101. doi: 10.1016/j.dnarep.2017.06.011. Epub 2017 Jun 9. DNA Repair (Amst). 2017. PMID: 28624371 Review.
-
Histone modifications and DNA double-strand break repair after exposure to ionizing radiations.Radiat Res. 2013 Apr;179(4):383-92. doi: 10.1667/RR3308.2. Epub 2013 Feb 1. Radiat Res. 2013. PMID: 23373901 Free PMC article. Review.
-
The relationship between histone posttranslational modification and DNA damage signaling and repair.Int J Radiat Biol. 2019 Apr;95(4):382-393. doi: 10.1080/09553002.2018.1516911. Epub 2018 Sep 25. Int J Radiat Biol. 2019. PMID: 30252564
-
E3 Ubiquitin Ligases RNF20 and RNF40 Are Required for Double-Stranded Break (DSB) Repair: Evidence for Monoubiquitination of Histone H2B Lysine 120 as a Novel Axis of DSB Signaling and Repair.Mol Cell Biol. 2019 Apr 2;39(8):e00488-18. doi: 10.1128/MCB.00488-18. Print 2019 Apr 15. Mol Cell Biol. 2019. PMID: 30692271 Free PMC article.
Cited by
-
MicroRNA-Mediated Regulation of Histone-Modifying Enzymes in Cancer: Mechanisms and Therapeutic Implications.Biomolecules. 2023 Oct 28;13(11):1590. doi: 10.3390/biom13111590. Biomolecules. 2023. PMID: 38002272 Free PMC article. Review.
-
Targeting the p53 signaling pathway in cancers: Molecular mechanisms and clinical studies.MedComm (2020). 2023 May 28;4(3):e288. doi: 10.1002/mco2.288. eCollection 2023 Jun. MedComm (2020). 2023. PMID: 37256211 Free PMC article. Review.
-
DNA Damage Response Alterations in Ovarian Cancer: From Molecular Mechanisms to Therapeutic Opportunities.Cancers (Basel). 2023 Jan 10;15(2):448. doi: 10.3390/cancers15020448. Cancers (Basel). 2023. PMID: 36672401 Free PMC article. Review.
-
DoUBLing up: ubiquitin and ubiquitin-like proteases in genome stability.Biochem J. 2024 Apr 10;481(7):515-545. doi: 10.1042/BCJ20230284. Biochem J. 2024. PMID: 38572758 Free PMC article. Review.
-
Noval advance of histone modification in inflammatory skin diseases and related treatment methods.Front Immunol. 2024 Jan 3;14:1286776. doi: 10.3389/fimmu.2023.1286776. eCollection 2023. Front Immunol. 2024. PMID: 38235133 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

