Mechanistic Interplay between HIV-1 Reverse Transcriptase Enzyme Kinetics and Host SAMHD1 Protein: Viral Myeloid-Cell Tropism and Genomic Mutagenesis
- PMID: 35893688
- PMCID: PMC9331428
- DOI: 10.3390/v14081622
Mechanistic Interplay between HIV-1 Reverse Transcriptase Enzyme Kinetics and Host SAMHD1 Protein: Viral Myeloid-Cell Tropism and Genomic Mutagenesis
Abstract
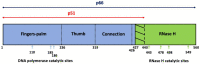
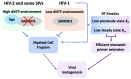
Human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) has been the primary interest among studies on antiviral discovery, viral replication kinetics, drug resistance, and viral evolution. Following infection and entry into target cells, the HIV-1 core disassembles, and the viral RT concomitantly converts the viral RNA into double-stranded proviral DNA, which is integrated into the host genome. The successful completion of the viral life cycle highly depends on the enzymatic DNA polymerase activity of RT. Furthermore, HIV-1 RT has long been known as an error-prone DNA polymerase due to its lack of proofreading exonuclease properties. Indeed, the low fidelity of HIV-1 RT has been considered as one of the key factors in the uniquely high rate of mutagenesis of HIV-1, which leads to efficient viral escape from immune and therapeutic antiviral selective pressures. Interestingly, a series of studies on the replication kinetics of HIV-1 in non-dividing myeloid cells and myeloid specific host restriction factor, SAM domain, and HD domain-containing protein, SAMHD1, suggest that the myeloid cell tropism and high rate of mutagenesis of HIV-1 are mechanistically connected. Here, we review not only HIV-1 RT as a key antiviral target, but also potential evolutionary and mechanistic crosstalk among the unique enzymatic features of HIV-1 RT, the replication kinetics of HIV-1, cell tropism, viral genetic mutation, and host SAMHD1 protein.
Keywords: SAMHD1; antiretroviral therapy; cell tropism; drug resistance; human immunodeficiency virus type 1; mutation; retrovirus; reverse transcriptase.
Conflict of interest statement
The authors declare no conflict of interest, and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
SAMHD1 specifically affects the antiviral potency of thymidine analog HIV reverse transcriptase inhibitors.Antimicrob Agents Chemother. 2014 Aug;58(8):4804-13. doi: 10.1128/AAC.03145-14. Epub 2014 Jun 9. Antimicrob Agents Chemother. 2014. PMID: 24913159 Free PMC article.
-
Cell-to-Cell Spreading of HIV-1 in Myeloid Target Cells Escapes SAMHD1 Restriction.mBio. 2019 Nov 19;10(6):e02457-19. doi: 10.1128/mBio.02457-19. mBio. 2019. PMID: 31744918 Free PMC article.
-
A Novel Leu92 Mutant of HIV-1 Reverse Transcriptase with a Selective Deficiency in Strand Transfer Causes a Loss of Viral Replication.J Virol. 2015 Aug;89(16):8119-29. doi: 10.1128/JVI.00809-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995261 Free PMC article.
-
Nucleoside-analog resistance mutations in HIV-1 reverse transcriptase and their influence on polymerase fidelity and viral mutation rates.Int J Biochem Cell Biol. 2004 Sep;36(9):1716-34. doi: 10.1016/j.biocel.2004.02.025. Int J Biochem Cell Biol. 2004. PMID: 15183340 Review.
-
Human Immunodeficiency Virus type 1 reverse transcriptase.Biol Chem Hoppe Seyler. 1996 Feb;377(2):97-120. Biol Chem Hoppe Seyler. 1996. PMID: 8868066 Review.
Cited by
-
Macrophages: Key Cellular Players in HIV Infection and Pathogenesis.Viruses. 2024 Feb 13;16(2):288. doi: 10.3390/v16020288. Viruses. 2024. PMID: 38400063 Free PMC article. Review.
-
Antiretroviral Treatment of HIV-2 Infection: Available Drugs, Resistance Pathways, and Promising New Compounds.Int J Mol Sci. 2023 Mar 21;24(6):5905. doi: 10.3390/ijms24065905. Int J Mol Sci. 2023. PMID: 36982978 Free PMC article. Review.
-
Fighting nature with nature: antiviral compounds that target retroviruses.Arch Microbiol. 2024 Feb 28;206(3):130. doi: 10.1007/s00203-024-03846-3. Arch Microbiol. 2024. PMID: 38416180 Review.
-
Unveiling the Connection: Viral Infections and Genes in dNTP Metabolism.Viruses. 2024 Sep 3;16(9):1412. doi: 10.3390/v16091412. Viruses. 2024. PMID: 39339888 Free PMC article. Review.
References
-
- Kennedy E.M., Gavegnano C., Nguyen L., Slater R., Lucas A., Fromentin E., Schinazi R.F., Kim B. Ribonucleoside triphosphates as substrate of human immunodeficiency virus type 1 reverse transcriptase in human macrophages. J. Biol. Chem. 2010;285:39380–39391. doi: 10.1074/jbc.M110.178582. - DOI - PMC - PubMed
-
- Diamond T.L., Roshal M., Jamburuthugoda V.K., Reynolds H.M., Merriam A.R., Lee K.Y., Balakrishnan M., Bambara R.A., Planelles V., Dewhurst S., et al. Macrophage tropism of HIV-1 depends on efficient cellular dNTP utilization by reverse transcriptase. J. Biol. Chem. 2004;279:51545–51553. doi: 10.1074/jbc.M408573200. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

