Leukocyte Telomere Length Variability as a Potential Biomarker in Patients with PolyQ Diseases
- PMID: 35892638
- PMCID: PMC9332235
- DOI: 10.3390/antiox11081436
Leukocyte Telomere Length Variability as a Potential Biomarker in Patients with PolyQ Diseases
Abstract
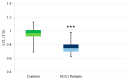
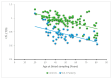
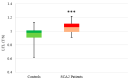
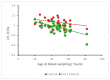
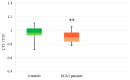
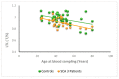
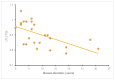
SCA1, SCA2, and SCA3 are the most common forms of SCAs among the polyglutamine disorders, which include Huntington's Disease (HD). We investigated the relationship between leukocyte telomere length (LTL) and the phenotype of SCA1, SCA2, and SCA3, comparing them with HD. The results showed that LTL was significantly reduced in SCA1 and SCA3 patients, while LTL was significantly longer in SCA2 patients. A significant negative relationship between LTL and age was observed in SCA1 but not in SCA2 subjects. LTL of SCA3 patients depend on both patient's age and disease duration. The number of CAG repeats did not affect LTL in the three SCAs. Since LTL is considered an indirect marker of an inflammatory response and oxidative damage, our data suggest that in SCA1 inflammation is present already at an early stage of disease similar to in HD, while in SCA3 inflammation and impaired antioxidative processes are associated with disease progression. Interestingly, in SCA2, contrary to SCA1 and SCA3, the length of leukocyte telomeres does not reduce with age. We have observed that SCAs and HD show a differing behavior in LTL for each subtype, which could constitute relevant biomarkers if confirmed in larger cohorts and longitudinal studies.
Keywords: biomarkers; leukocyte telomere length; neurodegenerative diseases; spinocerebellar ataxias.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
"I've Spent My Whole Life Striving to Be Normal": Internalized Stigma and Perceived Impact of Diagnosis in Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):423-436. doi: 10.1089/aut.2022.0066. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116050 Free PMC article.
-
Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review.
Cited by
-
Leukocyte Telomere Length as Potential Biomarker of HD Progression: A Follow-Up Study.Int J Mol Sci. 2022 Nov 3;23(21):13449. doi: 10.3390/ijms232113449. Int J Mol Sci. 2022. PMID: 36362235 Free PMC article.
-
Maternal stress-induced changes in adolescent and adult offspring: Neurobehavioural improvement and telomere maintenance.Heliyon. 2023 Sep 21;9(10):e20385. doi: 10.1016/j.heliyon.2023.e20385. eCollection 2023 Oct. Heliyon. 2023. PMID: 37767490 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

