Overview of Neutralization Assays and International Standard for Detecting SARS-CoV-2 Neutralizing Antibody
- PMID: 35891540
- PMCID: PMC9322699
- DOI: 10.3390/v14071560
Overview of Neutralization Assays and International Standard for Detecting SARS-CoV-2 Neutralizing Antibody
Abstract
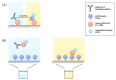
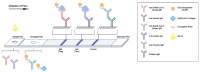
We aimed to review the existing literature on the different types of neutralization assays and international standards for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We comprehensively summarized the serological assays for detecting neutralizing antibodies against SARS-CoV-2 and demonstrated the importance of an international standard for calibrating the measurement of neutralizing antibodies. Following the coronavirus disease outbreak in December 2019, there was an urgent demand to detect neutralizing antibodies in patients or vaccinated people to monitor disease outcomes and determine vaccine efficacy. Therefore, many approaches were developed to detect neutralizing antibodies against SARS-CoV-2, such as microneutralization assay, SARS-CoV-2 pseudotype virus assay, enzyme-linked immunosorbent assay (ELISA), and rapid lateral flow assay. Given the many types of serological assays for quantifying the neutralizing antibody titer, the comparison of different assay results is a challenge. In 2020, the World Health Organization proposed the first international standard as a common unit to define neutralizing antibody titer and antibody responses against SARS-CoV-2. These standards are useful for comparing the results of different assays and laboratories.
Keywords: international standard for SARS-CoV-2; neutralizing antibody detection; serological assays.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
An Assessment of Serological Assays for SARS-CoV-2 as Surrogates for Authentic Virus Neutralization.Microbiol Spectr. 2021 Oct 31;9(2):e0105921. doi: 10.1128/Spectrum.01059-21. Epub 2021 Oct 27. Microbiol Spectr. 2021. PMID: 34704832 Free PMC article.
-
Evaluating Humoral Immunity against SARS-CoV-2: Validation of a Plaque-Reduction Neutralization Test and a Multilaboratory Comparison of Conventional and Surrogate Neutralization Assays.Microbiol Spectr. 2021 Dec 22;9(3):e0088621. doi: 10.1128/Spectrum.00886-21. Epub 2021 Nov 17. Microbiol Spectr. 2021. PMID: 34787495 Free PMC article.
-
Quantifying Neutralizing Antibodies in Patients with COVID-19 by a Two-Variable Generalized Additive Model.mSphere. 2022 Feb 23;7(1):e0088321. doi: 10.1128/msphere.00883-21. Epub 2022 Feb 2. mSphere. 2022. PMID: 35107336 Free PMC article.
-
Research progress in methods for detecting neutralizing antibodies against SARS-CoV-2.Anal Biochem. 2023 Jul 15;673:115199. doi: 10.1016/j.ab.2023.115199. Epub 2023 May 29. Anal Biochem. 2023. PMID: 37257735 Free PMC article. Review.
-
Alternative Methods to Detect Severe Acute Respiratory Syndrome Coronavirus 2 Antibodies.Clin Lab Med. 2022 Mar;42(1):57-73. doi: 10.1016/j.cll.2021.10.007. Epub 2021 Nov 3. Clin Lab Med. 2022. PMID: 35153048 Free PMC article. Review.
Cited by
-
Development of a Multispecies Double-Antigen Sandwich ELISA Using N and RBD Proteins to Detect Antibodies against SARS-CoV-2.Animals (Basel). 2023 Nov 12;13(22):3487. doi: 10.3390/ani13223487. Animals (Basel). 2023. PMID: 38003105 Free PMC article.
-
Centenarians, semi and supercentenarians, COVID-19 and Spanish flu: a serological assessment to gain insight into the resilience of older centenarians to COVID-19.Immun Ageing. 2024 Jun 27;21(1):44. doi: 10.1186/s12979-024-00450-3. Immun Ageing. 2024. PMID: 38937774 Free PMC article.
-
Correlation between pseudotyped virus and authentic virus neutralisation assays, a systematic review and meta-analysis of the literature.Front Immunol. 2023 Sep 18;14:1184362. doi: 10.3389/fimmu.2023.1184362. eCollection 2023. Front Immunol. 2023. PMID: 37790941 Free PMC article.
-
The Breadth of the Neutralizing Antibody Response to Original SARS-CoV-2 Infection is Linked to the Presence of Long COVID Symptoms.medRxiv [Preprint]. 2023 Mar 31:2023.03.30.23287923. doi: 10.1101/2023.03.30.23287923. medRxiv. 2023. Update in: J Med Virol. 2023 Nov;95(11):e29216. doi: 10.1002/jmv.29216. PMID: 37034660 Free PMC article. Updated. Preprint.
-
Snapshot of Anti-SARS-CoV-2 IgG Antibodies in COVID-19 Recovered Patients in Guinea.J Clin Med. 2024 May 17;13(10):2965. doi: 10.3390/jcm13102965. J Clin Med. 2024. PMID: 38792506 Free PMC article.
References
-
- Jiang C., Wang Y., Hu M., Wen L., Wen C., Wang Y., Zhu W., Tai S., Jiang Z., Xiao K., et al. Antibody seroconversion in asymptomatic and symptomatic patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Transl. Immunol. 2020;9:e1182. doi: 10.1002/cti2.1182. - DOI - PMC - PubMed
-
- Catry E., Jacqmin H., Dodemont M., Saad Albichr I., Lardinois B., de Fays B., Delaere B., Closset M., Laurent T., Denis O., et al. Analytical and clinical evaluation of four commercial SARS-CoV-2 serological immunoassays in hospitalized patients and ambulatory individuals. J. Virol. Methods. 2021;289:114060. doi: 10.1016/j.jviromet.2020.114060. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

