Increased EBV DNAemia after Anti-SARS-CoV-2 Vaccination in Solid Organ Transplants
- PMID: 35891156
- PMCID: PMC9325163
- DOI: 10.3390/vaccines10070992
Increased EBV DNAemia after Anti-SARS-CoV-2 Vaccination in Solid Organ Transplants
Abstract
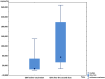
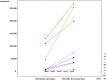
The reactivation of latent viruses during SARS-CoV-2 infection is well recognized, and coinfection with Epstein−Barr virus (EBV) has been associated with severe clinical cases of COVID-19 infection. In transplant patients, EBV infection presents a significant challenge. Assessing the potential impact of SARS-CoV-2 vaccinations on EBV infections in stable kidney and liver transplant recipients was the objective of our study. Ten solid-organ-transplant (SOT) patients (eight kidney and two liver) vaccinated with standard doses of mRNA COVID-19 vaccines were included. EBV DNA viral load measurements were conducted prior to the vaccination and during a follow-up period (at the first month and after six months) after the second vaccine dose. After the second dose, a significant increase in median viremia was observed (p < 0.01) in 9 patients, and in one patient, the reactivation of EBV infection was found. Six months later, the median viremia decreased significantly (p < 0.05). The EBV viral load should be closely monitored as it could lead to the earlier diagnosis and treatment of EBV-related complications. Despite experiencing a decrease in the viral load six months post-vaccination, some patients still had a viral load over the baseline, which increased the risk of potential complications.
Keywords: COVID-19; Epstein–Barr virus; solid organ transplant; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
EBV Reactivation in Transplant Recipients following SARS-CoV-2 Infection: A Retrospective Study.Pathogens. 2023 Dec 10;12(12):1435. doi: 10.3390/pathogens12121435. Pathogens. 2023. PMID: 38133317 Free PMC article.
-
Current preventive strategies and management of Epstein-Barr virus-related post-transplant lymphoproliferative disease in solid organ transplantation in Europe. Results of the ESGICH Questionnaire-based Cross-sectional Survey.Clin Microbiol Infect. 2015 Jun;21(6):604.e1-9. doi: 10.1016/j.cmi.2015.02.002. Epub 2015 Feb 14. Clin Microbiol Infect. 2015. PMID: 25686696
-
Epstein-Barr virus-related post-transplant lymphoproliferative disorder in solid organ transplant recipients.Clin Microbiol Infect. 2014 Sep;20 Suppl 7:109-18. doi: 10.1111/1469-0691.12534. Clin Microbiol Infect. 2014. PMID: 24475976 Review.
-
[Epstein Barr viral load monitoring in mononuclear lymphocytes and serum of renal transplant recipients using a quantitative PCR protocol].G Ital Nefrol. 2003 Mar-Apr;20(2):170-5. G Ital Nefrol. 2003. PMID: 12746803 Italian.
-
Epstein-Barr virus associated smooth muscle tumors in solid organ transplant recipients: Incidence over 31 years at a single institution and review of the literature.Transpl Infect Dis. 2019 Feb;21(1):e13010. doi: 10.1111/tid.13010. Epub 2018 Oct 25. Transpl Infect Dis. 2019. PMID: 30298678 Review.
Cited by
-
EBV Reactivation in Transplant Recipients following SARS-CoV-2 Infection: A Retrospective Study.Pathogens. 2023 Dec 10;12(12):1435. doi: 10.3390/pathogens12121435. Pathogens. 2023. PMID: 38133317 Free PMC article.
-
SARS-CoV-2 Induced Herpes Virus Reactivations and Related Implications in Oncohematology: When Lymphocytopenia Sets in and Immunosurveillance Drops Out.Microorganisms. 2023 Sep 1;11(9):2223. doi: 10.3390/microorganisms11092223. Microorganisms. 2023. PMID: 37764067 Free PMC article.
-
Herpesviruses reactivation following COVID-19 vaccination: a systematic review and meta-analysis.Eur J Med Res. 2023 Aug 10;28(1):278. doi: 10.1186/s40001-023-01238-9. Eur J Med Res. 2023. PMID: 37559096 Free PMC article. Review.
-
Safety of COVID-19 Vaccines in Patients with Autoimmune Diseases, in Patients with Cardiac Issues, and in the Healthy Population.Pathogens. 2023 Feb 2;12(2):233. doi: 10.3390/pathogens12020233. Pathogens. 2023. PMID: 36839505 Free PMC article. Review.
-
Saliva antibody-fingerprint of reactivated latent viruses after mild/asymptomatic COVID-19 is unique in patients with myalgic-encephalomyelitis/chronic fatigue syndrome.Front Immunol. 2022 Oct 20;13:949787. doi: 10.3389/fimmu.2022.949787. eCollection 2022. Front Immunol. 2022. PMID: 36341457 Free PMC article.
References
-
- Hall V.G., Ferreira V.H., Ierullo M., Ku T., Marinelli T., Majchrzak-Kita B., Yousuf A., Kulasingam V., Humar A., Kumar D. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am. J. Transplant. 2021;21:3980–3989. doi: 10.1111/ajt.16766. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

