Developing New Treatments for COVID-19 through Dual-Action Antiviral/Anti-Inflammatory Small Molecules and Physiologically Based Pharmacokinetic Modeling
- PMID: 35887353
- PMCID: PMC9325261
- DOI: 10.3390/ijms23148006
Developing New Treatments for COVID-19 through Dual-Action Antiviral/Anti-Inflammatory Small Molecules and Physiologically Based Pharmacokinetic Modeling
Abstract
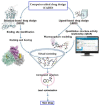
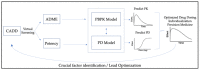
Broad-spectrum antiviral agents that are effective against many viruses are difficult to develop, as the key molecules, as well as the biochemical pathways by which they cause infection, differ largely from one virus to another. This was more strongly highlighted by the COVID-19 pandemic, which found health systems all over the world largely unprepared and proved that the existing armamentarium of antiviral agents is not sufficient to address viral threats with pandemic potential. The clinical protocols for the treatment of COVID-19 are currently based on the use of inhibitors of the inflammatory cascade (dexamethasone, baricitinib), or inhibitors of the cytopathic effect of the virus (monoclonal antibodies, molnupiravir or nirmatrelvir/ritonavir), using different agents. There is a critical need for an expanded armamentarium of orally bioavailable small-molecular medicinal agents, including those that possess dual antiviral and anti-inflammatory (AAI) activity that would be readily available for the early treatment of mild to moderate COVID-19 in high-risk patients. A multidisciplinary approach that involves the use of in silico screening tools to identify potential drug targets of an emerging pathogen, as well as in vitro and in vivo models for the determination of a candidate drug's efficacy and safety, are necessary for the rapid and successful development of antiviral agents with potentially dual AAI activity. Characterization of candidate AAI molecules with physiologically based pharmacokinetics (PBPK) modeling would provide critical data for the accurate dosing of new therapeutic agents against COVID-19. This review analyzes the dual mechanisms of AAI agents with potential anti-SARS-CoV-2 activity and discusses the principles of PBPK modeling as a conceptual guide to develop new pharmacological modalities for the treatment of COVID-19.
Keywords: COVID-19; PBPK modeling; antiviral agents; dual action; molecular docking.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures
Similar articles
-
Molnupiravir and Its Antiviral Activity Against COVID-19.Front Immunol. 2022 Apr 4;13:855496. doi: 10.3389/fimmu.2022.855496. eCollection 2022. Front Immunol. 2022. PMID: 35444647 Free PMC article. Review.
-
4'-Fluorouridine Is a Broad-Spectrum Orally Available First-Line Antiviral That May Improve Pandemic Preparedness.DNA Cell Biol. 2022 Aug;41(8):699-704. doi: 10.1089/dna.2022.0312. Epub 2022 Jul 5. DNA Cell Biol. 2022. PMID: 35788144 Free PMC article. Review.
-
COVID-19 treatments approved in the European Union and clinical recommendations for the management of non-hospitalized and hospitalized patients.Ann Med. 2022 Dec;54(1):2856-2860. doi: 10.1080/07853890.2022.2133162. Ann Med. 2022. PMID: 36259490 Free PMC article. Review.
-
[Current and future therapeutic options for COVID-19].Ned Tijdschr Geneeskd. 2022 Jul 21;166:D6675. Ned Tijdschr Geneeskd. 2022. PMID: 36036702 Dutch.
-
Dual action anti-inflammatory/antiviral isoquinoline alkaloids as potent naturally occurring anti-SARS-CoV-2 agents: A combined pharmacological and medicinal chemistry perspective.Phytother Res. 2023 May;37(5):2168-2186. doi: 10.1002/ptr.7833. Epub 2023 Apr 11. Phytother Res. 2023. PMID: 37039761 Review.
Cited by
-
Identification of potential inhibitors for drug-resistant EGFR mutations in non-small cell lung cancer using whole exome sequencing data.Front Pharmacol. 2024 Jul 25;15:1428158. doi: 10.3389/fphar.2024.1428158. eCollection 2024. Front Pharmacol. 2024. PMID: 39130636 Free PMC article.
-
Enzymes and Enzyme Inhibitors-Applications in Medicine and Diagnosis.Int J Mol Sci. 2023 Mar 9;24(6):5245. doi: 10.3390/ijms24065245. Int J Mol Sci. 2023. PMID: 36982319 Free PMC article.
-
Viral Factors in Modulation of Host Immune Response: A Route to Novel Antiviral Agents and New Therapeutic Approaches.Int J Mol Sci. 2024 Aug 29;25(17):9408. doi: 10.3390/ijms25179408. Int J Mol Sci. 2024. PMID: 39273355 Free PMC article. Review.
References
-
- Coronavirus 2019-nCoV, CSSE Coronavirus 2019-nCoV Global Cases by Johns Hopkins CSSE. [(accessed on 15 December 2020)]. Available online: https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594....
-
- WHO Novel Coronavirus e China. 2020. [(accessed on 15 December 2020)]. Available online: https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/
-
- Farahani M., Niknam Z., Mohammadi Amirabad L., Amiri-Dashatan N., Koushki M., Nemati M., Danesh Pouya F., Rezaei-Tavirani M., Rasmi Y., Tayebi L. Molecular pathways involved in COVID-19 and potential pathway-based therapeutic targets. BioMed Pharmacother. 2022;145:112420. doi: 10.1016/j.biopha.2021.112420. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

