Recent Advances Regarding the Molecular Mechanisms of Triterpenic Acids: A Review (Part I)
- PMID: 35887090
- PMCID: PMC9322890
- DOI: 10.3390/ijms23147740
Recent Advances Regarding the Molecular Mechanisms of Triterpenic Acids: A Review (Part I)
Abstract
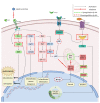
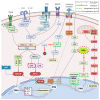
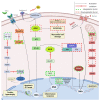
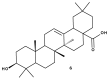
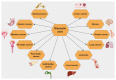
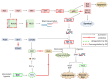
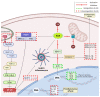
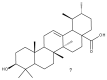
Triterpenic acids are phytocompounds with a widespread range of biological activities that have been the subject of numerous in vitro and in vivo studies. However, their underlying mechanisms of action in various pathologies are not completely elucidated. The current review aims to summarize the most recent literature, published in the last five years, regarding the mechanism of action of three triterpenic acids (asiatic acid, oleanolic acid, and ursolic acid), corelated with different biological activities such as anticancer, anti-inflammatory, antidiabetic, cardioprotective, neuroprotective, hepatoprotective, and antimicrobial. All three discussed compounds share several mechanisms of action, such as the targeted modulation of the PI3K/AKT, Nrf2, NF-kB, EMT, and JAK/STAT3 signaling pathways, while other mechanisms that proved to only be specific for a part of the triterpenic acids discussed, such as the modulation of Notch, Hippo, and MALAT1/miR-206/PTGS1 signaling pathway, were highlighted as well. This paper stands as the first part in our literature study on the topic, which will be followed by a second part focusing on other triterpenic acids of therapeutic value.
Keywords: asiatic acid; molecular mechanism; molecular target; oleanolic acid; triterpenic acid; ursolic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Recent Advances Regarding the Molecular Mechanisms of Triterpenic Acids: A Review (Part II).Int J Mol Sci. 2022 Aug 10;23(16):8896. doi: 10.3390/ijms23168896. Int J Mol Sci. 2022. PMID: 36012159 Free PMC article. Review.
-
Ursolic and Oleanolic Acids: Plant Metabolites with Neuroprotective Potential.Int J Mol Sci. 2021 Apr 27;22(9):4599. doi: 10.3390/ijms22094599. Int J Mol Sci. 2021. PMID: 33925641 Free PMC article. Review.
-
Antioxidative and antiinflammatory activities of asiatic acid, glycyrrhizic acid, and oleanolic acid in human bronchial epithelial cells.J Agric Food Chem. 2015 Apr 1;63(12):3196-204. doi: 10.1021/acs.jafc.5b00102. Epub 2015 Mar 24. J Agric Food Chem. 2015. Retraction in: J Agric Food Chem. 2017 Apr 19;65(15):3251. doi: 10.1021/acs.jafc.7b01473 PMID: 25779760 Retracted.
-
In vivo anti-malarial activity and toxicity studies of triterpenic esters isolated form Keetia leucantha and crude extracts.Malar J. 2017 Oct 10;16(1):406. doi: 10.1186/s12936-017-2054-y. Malar J. 2017. PMID: 29017554 Free PMC article.
-
Treatment with triterpenic fraction purified from Baccharis uncinella leaves inhibits Leishmania (Leishmania) amazonensis spreading and improves Th1 immune response in infected mice.Parasitol Res. 2014 Jan;113(1):333-9. doi: 10.1007/s00436-013-3659-x. Epub 2013 Oct 31. Parasitol Res. 2014. PMID: 24173812
Cited by
-
The Anti-Melanoma Effect of Betulinic Acid Functionalized Gold Nanoparticles: A Mechanistic In Vitro Approach.Pharmaceuticals (Basel). 2022 Nov 5;15(11):1362. doi: 10.3390/ph15111362. Pharmaceuticals (Basel). 2022. PMID: 36355533 Free PMC article.
-
Ursolic acid: a natural modulator of signaling networks in different cancers.Cancer Cell Int. 2022 Dec 10;22(1):399. doi: 10.1186/s12935-022-02804-7. Cancer Cell Int. 2022. PMID: 36496432 Free PMC article. Review.
-
The Antimelanoma Biological Assessment of Triterpenic Acid Functionalized Gold Nanoparticles.Molecules. 2023 Jan 3;28(1):421. doi: 10.3390/molecules28010421. Molecules. 2023. PMID: 36615613 Free PMC article.
-
Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health.Molecules. 2022 Nov 13;27(22):7823. doi: 10.3390/molecules27227823. Molecules. 2022. PMID: 36431924 Free PMC article. Review.
-
Systematic Review of Chemical Compounds with Immunomodulatory Action Isolated from African Medicinal Plants.Molecules. 2024 Apr 26;29(9):2010. doi: 10.3390/molecules29092010. Molecules. 2024. PMID: 38731500 Free PMC article. Review.
References
-
- D’yakonov V.A., Dzhemileva L.U., Dzhemilev U.M. Advances in the Chemistry of Natural and Semisynthetic Topoisomerase I/II Inhibitors. Stud. Nat. Prod. Chem. 2017;54:21–86.
-
- Ghosh G., Subudhi B., Mishra S.K. Antihyperglycemic activity of root bark of Polyalthia longifolia var. pendula and aerial parts of Sida rhombifolia Linn. and its relationship with antioxidant property. Asian J. Chem. 2011;23:141–144.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

