Mechanical Properties of the Extracellular Environment of Human Brain Cells Drive the Effectiveness of Drugs in Fighting Central Nervous System Cancers
- PMID: 35884733
- PMCID: PMC9313046
- DOI: 10.3390/brainsci12070927
Mechanical Properties of the Extracellular Environment of Human Brain Cells Drive the Effectiveness of Drugs in Fighting Central Nervous System Cancers
Abstract
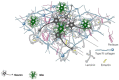
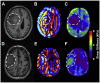
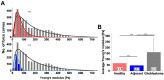
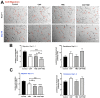
The evaluation of nanomechanical properties of tissues in health and disease is of increasing interest to scientists. It has been confirmed that these properties, determined in part by the composition of the extracellular matrix, significantly affect tissue physiology and the biological behavior of cells, mainly in terms of their adhesion, mobility, or ability to mutate. Importantly, pathophysiological changes that determine disease development within the tissue usually result in significant changes in tissue mechanics that might potentially affect the drug efficacy, which is important from the perspective of development of new therapeutics, since most of the currently used in vitro experimental models for drug testing do not account for these properties. Here, we provide a summary of the current understanding of how the mechanical properties of brain tissue change in pathological conditions, and how the activity of the therapeutic agents is linked to this mechanical state.
Keywords: brain; extra cellular matrix; glioblastoma; mechanical properties; rheology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Impact of microstructure on cell behavior and tissue mechanics in collagen and dermal decellularized extra-cellular matrices.Acta Biomater. 2022 Apr 15;143:100-114. doi: 10.1016/j.actbio.2022.02.035. Epub 2022 Feb 27. Acta Biomater. 2022. PMID: 35235868
-
Macromolecular crowding: chemistry and physics meet biology (Ascona, Switzerland, 10-14 June 2012).Phys Biol. 2013 Aug;10(4):040301. doi: 10.1088/1478-3975/10/4/040301. Epub 2013 Aug 2. Phys Biol. 2013. PMID: 23912807
-
Cell-matrix mechanical interaction in electrospun polymeric scaffolds for tissue engineering: Implications for scaffold design and performance.Acta Biomater. 2017 Mar 1;50:41-55. doi: 10.1016/j.actbio.2016.12.034. Epub 2016 Dec 21. Acta Biomater. 2017. PMID: 28011142 Review.
-
Tissue mechanics, an important regulator of development and disease.Philos Trans R Soc Lond B Biol Sci. 2019 Aug 19;374(1779):20180215. doi: 10.1098/rstb.2018.0215. Epub 2019 Jul 1. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31431174 Free PMC article. Review.
-
Modeling the mechanical stiffness of pancreatic ductal adenocarcinoma.Matrix Biol Plus. 2022 Mar 21;14:100109. doi: 10.1016/j.mbplus.2022.100109. eCollection 2022 Jun. Matrix Biol Plus. 2022. PMID: 35399702 Free PMC article.
Cited by
-
Extracellular Matrix Components and Mechanosensing Pathways in Health and Disease.Biomolecules. 2024 Sep 20;14(9):1186. doi: 10.3390/biom14091186. Biomolecules. 2024. PMID: 39334952 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

