Inhibition of NEDD8 NEDDylation induced apoptosis in acute myeloid leukemia cells via p53 signaling pathway
- PMID: 35880551
- PMCID: PMC9386570
- DOI: 10.1042/BSR20220994
Inhibition of NEDD8 NEDDylation induced apoptosis in acute myeloid leukemia cells via p53 signaling pathway
Abstract
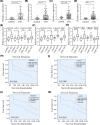
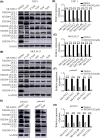
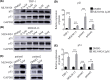
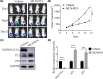
MLN4924 is a potent and selective small-molecule inhibitor of NEDD8-activating enzyme, which showed antitumor effect in several types of malignant tumor types. However, the mechanism of action of MLN4924 in acute myeloid leukemia (AML) requires further investigation. Real-time fluorescent quantitative polymerase chain reaction (RT-qPCR) was conducted to detect the mRNA levels of genes. Gene expression was knocked down by short hairpin RNA (shRNA). Moreover, the protein expression was detected by Western blotting (WB) assay. The proliferation and apoptosis of AML cells were measured by Cell Counting Kit-8 (CCK8) assay and flow cytometry (FCM). In the present study, we observed that the mRNA expression levels of NEDD8, UBA3, UBE2M and RBX1 in AML patients were up-regulated compared with healthy controls, which were correlated with worse overall survival (OS) of patients. Besides, knockdown of UBA3, UBE2M and RBX1 inhibited the NEDDylation of CULs and increased the protein expression of p53 and p21 in MOLM-13 cell line. In AML cells, MLN4924 inhibited cell proliferation, promoted cell apoptosis, and induced cell cycle arrest at the G2/M phase. As revealed by experiments in vivo and in vitro, the NEDDylation of CULs was significantly inhibited and the p53 signaling pathway was activated after MLN4924 treatment. So, we concluded that NEDD8, UBA3, UBE2M and RBX1 may serve as the prognostic biomarkers and novel therapeutic targets for AML. Inhibition of the NEDDylation pathway resulted in an anti-leukemia effect by activating the p53 signaling pathway.
Keywords: AML; MLN4924; NEDD8; p21; p53.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


Similar articles
-
Validation of NEDD8-conjugating enzyme UBC12 as a new therapeutic target in lung cancer.EBioMedicine. 2019 Jul;45:81-91. doi: 10.1016/j.ebiom.2019.06.005. Epub 2019 Jun 14. EBioMedicine. 2019. PMID: 31208947 Free PMC article.
-
Pharmacological targeting of miR-155 via the NEDD8-activating enzyme inhibitor MLN4924 (Pevonedistat) in FLT3-ITD acute myeloid leukemia.Leukemia. 2015 Oct;29(10):1981-92. doi: 10.1038/leu.2015.106. Epub 2015 May 14. Leukemia. 2015. PMID: 25971362 Free PMC article.
-
A first-in-class inhibitor, MLN4924 (pevonedistat), induces cell-cycle arrest, senescence, and apoptosis in human renal cell carcinoma by suppressing UBE2M-dependent neddylation modification.Cancer Chemother Pharmacol. 2018 Jun;81(6):1083-1093. doi: 10.1007/s00280-018-3582-z. Epub 2018 Apr 17. Cancer Chemother Pharmacol. 2018. PMID: 29667067
-
The Double-Edged Effects of MLN4924: Rethinking Anti-Cancer Drugs Targeting the Neddylation Pathway.Biomolecules. 2024 Jun 21;14(7):738. doi: 10.3390/biom14070738. Biomolecules. 2024. PMID: 39062453 Free PMC article. Review.
-
Targeting DCN1-UBC12 Protein-Protein Interaction for Regulation of Neddylation Pathway.Adv Exp Med Biol. 2020;1217:349-362. doi: 10.1007/978-981-15-1025-0_20. Adv Exp Med Biol. 2020. PMID: 31898237 Review.
Cited by
-
Targeting NEDD8-activating enzyme for cancer therapy: developments, clinical trials, challenges and future research directions.J Hematol Oncol. 2023 Jul 31;16(1):87. doi: 10.1186/s13045-023-01485-7. J Hematol Oncol. 2023. PMID: 37525282 Free PMC article. Review.
-
UBA3 promotes the occurrence and metastasis of intrahepatic cholangiocarcinoma through MAPK signaling pathway.Acta Biochim Biophys Sin (Shanghai). 2024 Feb 25;56(2):199-209. doi: 10.3724/abbs.2024014. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38298057 Free PMC article.
-
METTL protein family: focusing on the occurrence, progression and treatment of cancer.Biomark Res. 2024 Sep 17;12(1):105. doi: 10.1186/s40364-024-00652-3. Biomark Res. 2024. PMID: 39289775 Free PMC article. Review.
-
Promising Therapeutic Strategies for Hematologic Malignancies: Innovations and Potential.Molecules. 2024 Sep 9;29(17):4280. doi: 10.3390/molecules29174280. Molecules. 2024. PMID: 39275127 Free PMC article. Review.
-
ncRNAs Orchestrate Chemosensitivity Induction by Neddylation Blockades.Cancers (Basel). 2024 Feb 18;16(4):825. doi: 10.3390/cancers16040825. Cancers (Basel). 2024. PMID: 38398217 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

