Agonist concentration-dependent changes in FPR1 conformation lead to biased signaling for selective activation of phagocyte functions
- PMID: 35878025
- PMCID: PMC9351494
- DOI: 10.1073/pnas.2201249119
Agonist concentration-dependent changes in FPR1 conformation lead to biased signaling for selective activation of phagocyte functions
Abstract
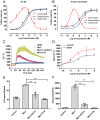
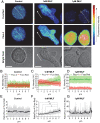
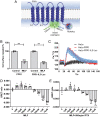
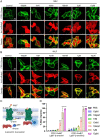
The bacteria-derived formyl peptide fMet-Leu-Phe (fMLF) is a potent chemoattractant of phagocytes that induces chemotaxis at subnanomolar concentrations. At higher concentrations, fMLF inhibits chemotaxis while stimulating degranulation and superoxide production, allowing phagocytes to kill invading bacteria. How an agonist activates distinct cellular functions at different concentrations remains unclear. Using a bioluminescence resonance energy transfer-based FPR1 biosensor, we found that fMLF at subnanomolar and micromolar concentrations induced distinct conformational changes in FPR1, a Gi-coupled chemoattractant receptor that activates various phagocyte functions. Neutrophil-like HL-60 cells exposed to subnanomolar concentrations of fMLF polarized rapidly and migrated along a chemoattractant concentration gradient. These cells also developed an intracellular Ca2+ concentration gradient. In comparison, high nanomolar and micromolar concentrations of fMLF triggered the PLC-β/diacyl glycerol/inositol trisphosphate pathway downstream of the heterotrimeric Gi proteins, leading to Ca2+ mobilization from intracellular stores and Ca2+ influx from extracellular milieu. A robust and uniform rise in cytoplasmic Ca2+ level was required for degranulation and superoxide production but disrupted cytoplasmic Ca2+ concentration gradient and inhibited chemotaxis. In addition, elevated ERK1/2 phosphorylation and β-arrestin2 membrane translocation were associated with diminished chemotaxis in the presence of fMLF above 1 nM. These findings suggest a mechanism for FPR1 agonist concentration-dependent signaling that leads to a switch from migration to bactericidal activities in phagocytes.
Keywords: GPCRs; biased signaling; calcium mobilization; phagocytes.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
Functional selective FPR1 signaling in favor of an activation of the neutrophil superoxide generating NOX2 complex.J Leukoc Biol. 2021 Jun;109(6):1105-1120. doi: 10.1002/JLB.2HI0520-317R. Epub 2020 Oct 11. J Leukoc Biol. 2021. PMID: 33040403 Free PMC article.
-
Design, synthesis and characterization of fMLF-mimicking AApeptides.Chembiochem. 2014 Nov 3;15(16):2420-6. doi: 10.1002/cbic.201402396. Epub 2014 Sep 15. Chembiochem. 2014. PMID: 25224835 Free PMC article.
-
The formyl peptide fMLF primes platelet activation and augments thrombus formation.J Thromb Haemost. 2019 Jul;17(7):1120-1133. doi: 10.1111/jth.14466. Epub 2019 May 24. J Thromb Haemost. 2019. PMID: 31033193 Free PMC article.
-
Signal transduction in cells following binding of chemoattractants to membrane receptors.Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;55(2):65-80. doi: 10.1007/BF02896561. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988. PMID: 2901161 Review.
-
Dynamics of human neutrophil receptors for the chemoattractant fmet-leu-phe.Agents Actions Suppl. 1983;12:290-308. doi: 10.1007/978-3-0348-9352-7_17. Agents Actions Suppl. 1983. PMID: 6301232 Review.
Cited by
-
Promiscuous Receptors and Neuroinflammation: The Formyl Peptide Class.Life (Basel). 2022 Dec 2;12(12):2009. doi: 10.3390/life12122009. Life (Basel). 2022. PMID: 36556373 Free PMC article. Review.
-
Increased expression of formyl peptide receptor-1 by basophils from patients with mastocytosis.J Allergy Clin Immunol Glob. 2024 Jul 3;3(4):100296. doi: 10.1016/j.jacig.2024.100296. eCollection 2024 Nov. J Allergy Clin Immunol Glob. 2024. PMID: 39148513 Free PMC article.
-
Stimulation of platelet P2Y1 receptors by different endogenous nucleotides leads to functional selectivity via biased signalling.Br J Pharmacol. 2024 Feb;181(4):564-579. doi: 10.1111/bph.16039. Epub 2023 Feb 13. Br J Pharmacol. 2024. PMID: 36694432 Free PMC article.
References
-
- Boulay F., Tardif M., Brouchon L., Vignais P., The human N-formylpeptide receptor. Characterization of two cDNA isolates and evidence for a new subfamily of G-protein-coupled receptors. Biochemistry 29, 11123–11133 (1990). - PubMed
-
- Marasco W. A., et al. , Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J. Biol. Chem. 259, 5430–5439 (1984). - PubMed
-
- Nathan C., Neutrophils and immunity: Challenges and opportunities. Nat. Rev. Immunol. 6, 173–182 (2006). - PubMed
-
- Kolaczkowska E., Kubes P., Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

