Inactivated COVID-19 vaccination does not affect in vitro fertilization outcomes in women
- PMID: 35876815
- PMCID: PMC9384590
- DOI: 10.1093/humrep/deac160
Inactivated COVID-19 vaccination does not affect in vitro fertilization outcomes in women
Abstract
Study question: Do inactivated coronavirus disease-2019 (COVID-19) vaccines affect IVF outcomes among the vaccine recipients?
Summary answer: The receipt of inactivated COVID-19 vaccines before ovarian stimulation has little effect on the outcomes of IVF, including ovarian stimulation outcomes, embryo development and pregnancy rates.
What is known already: Limited studies have reported that COVID-19 vaccines do not affect ovarian function, embryo development or pregnancy outcomes.
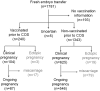
Study design, size, duration: This was a retrospective cohort study performed at the Third Affiliated Hospital of Guangzhou Medical University on 240 women vaccinated with either CoronaVac or Sinopharm COVID-19 before ovarian stimulation in the exposed group and 1343 unvaccinated women before ovarian stimulation in the unexposed group. All participants received fresh embryo transfers between 1 March 2021 and 15 September 2021. The included women were followed up until 12 weeks of gestation.
Participants/materials, setting, methods: Vaccination information of all subjects was followed up by a nurse, and the IVF data were obtained from the IVF data system. The following aspects were compared between the vaccinated and the unvaccinated groups: parameters of ovarian stimulation, embryo development and pregnancy rates. Regression analyses were performed to control for confounders of embryo development and pregnancy rates. Propensity score matching (PSM) was performed to balance the baseline parameters of the two groups. The primary outcome was the ongoing pregnancy rate.
Main results and the role of chance: Liner regression analysis revealed that the number of oocytes retrieved (regression coefficient (B) = -0.299, P = 0.264), embryos suitable for transfer (B = -0.203, P = 0.127) and blastocysts (B = -0.250, P = 0.105) were not associated with the status of vaccination before ovarian stimulation, after adjusting for the confounders. The ongoing pregnancy rate in the women of the vaccinated group was not significantly lower than that in the unvaccinated group (36.3% vs 40.7%, P = 0.199) (adjust odd ratio = 0.91, 95% CI = 0.68-1.22, P = 0.52). After PSM, the rates of ongoing pregnancy (36.0% vs 39.9%, P = 0.272), implantation (35.4% vs 38.3%, P = 0.325), biochemical pregnancy (47.3% vs 51.6%, P = 0.232), clinical pregnancy (44.4% vs 47.4%, P = 0.398) and early miscarriage (15.0% vs 12.1%, P = 0.399) were not significantly different between the vaccinated and the unvaccinated groups.
Limitations, reasons for caution: This is a retrospective study of women with infertility. The results from the present study warrant confirmation by prospective studies with a larger cohort.
Wider implications of the findings: This is the first study with a large sample size on the effect of inactivated COVID-19 vaccines on ongoing pregnancy rates of women undergoing IVF. The present results showed that vaccination has no detrimental effect on IVF outcomes. Therefore, women are recommended to receive COVID-19 vaccines before undergoing their IVF treatment.
Study funding/competing interest(s): This study was supported by the National Key Research and Development Program of China (No. 2018YFC1003803 to J.L.), the Guangzhou Science and Technology Plan Project (No. 202102010076 to H.L.) and the Medical Key Discipline of Guangzhou (2021-2023), as well as the Sino-German Center for Research Promotion Rapid Response Funding Call for Bilateral Collaborative Proposals between China and Germany in COVID-19 Related Research (No. C-0032 to Xingfei Pan). The authors declare no conflicts of interest.
Trial registration number: N/A.
Keywords: in vitro fertilization; COVID-19; embryo transfer; pregnancy; vaccination.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures

Similar articles
-
Inactivated Covid-19 vaccine did not undermine live birth and neonatal outcomes of women with frozen-thawed embryo transfer.Hum Reprod. 2022 Nov 24;37(12):2942-2951. doi: 10.1093/humrep/deac220. Hum Reprod. 2022. PMID: 36200874 Free PMC article.
-
Recent clomiphene citrate exposure does not impact subsequent clinical outcomes in single euploid frozen embryo transfer cycles.Hum Reprod. 2023 Jun 1;38(6):1151-1161. doi: 10.1093/humrep/dead072. Hum Reprod. 2023. PMID: 37075318
-
Ovulation triggering with hCG alone, GnRH agonist alone or in combination? A randomized controlled trial in advanced-age women undergoing IVF/ICSI cycles.Hum Reprod. 2022 Jul 30;37(8):1795-1805. doi: 10.1093/humrep/deac114. Hum Reprod. 2022. PMID: 35595223 Clinical Trial.
-
Does SARS Cov-2 infection affect the IVF outcome - A systematic review and meta-analysis.Eur J Obstet Gynecol Reprod Biol. 2024 Jan;292:147-157. doi: 10.1016/j.ejogrb.2023.11.027. Epub 2023 Nov 22. Eur J Obstet Gynecol Reprod Biol. 2024. PMID: 38006819 Review.
-
Effect of female coronavirus disease 2019 vaccination on assisted reproductive outcomes: a systematic review and meta-analysis.Fertil Steril. 2023 May;119(5):772-783. doi: 10.1016/j.fertnstert.2023.01.024. Epub 2023 Jan 23. Fertil Steril. 2023. PMID: 36702343 Free PMC article. Review.
Cited by
-
Effect of inactivated COVID-19 vaccination on pregnancy outcomes following frozen-thawed embryo transfer: A retrospective cohort study.Int Immunopharmacol. 2023 Jan;114:109552. doi: 10.1016/j.intimp.2022.109552. Epub 2022 Dec 9. Int Immunopharmacol. 2023. PMID: 36527882 Free PMC article.
-
COVID-19 Vaccines and Assisted Reproductive Techniques: A Systematic Review.J Pers Med. 2023 Aug 4;13(8):1232. doi: 10.3390/jpm13081232. J Pers Med. 2023. PMID: 37623482 Free PMC article. Review.
-
Pregnancy outcomes following natural conception and assisted reproduction treatment in women who received COVID-19 vaccination prior to conception: a population-based cohort study in China.Front Med (Lausanne). 2023 Oct 11;10:1250165. doi: 10.3389/fmed.2023.1250165. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37886353 Free PMC article.
-
Association Between COVID-19 Vaccination and Artificial Insemination Outcomes for Couples Experiencing Infertility.JAMA Netw Open. 2022 Dec 1;5(12):e2247216. doi: 10.1001/jamanetworkopen.2022.47216. JAMA Netw Open. 2022. PMID: 36525277 Free PMC article.
-
Endocrine system after 2 years of COVID-19 vaccines: A narrative review of the literature.Front Endocrinol (Lausanne). 2022 Nov 10;13:1027047. doi: 10.3389/fendo.2022.1027047. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36440218 Free PMC article. Review.
References
-
- Aharon D, Lederman M, Ghofranian A, Hernandez-Nieto C, Canon C, Hanley W, Gounko D, Lee JA, Stein D, Buyuk E. et al. In vitro fertilization and early pregnancy outcomes after coronavirus disease 2019 (COVID-19) vaccination. Obstet Gynecol 2022;139:490–497. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

