In Vivo ETosis of Human Eosinophils: The Ultrastructural Signature Captured by TEM in Eosinophilic Diseases
- PMID: 35874692
- PMCID: PMC9301467
- DOI: 10.3389/fimmu.2022.938691
In Vivo ETosis of Human Eosinophils: The Ultrastructural Signature Captured by TEM in Eosinophilic Diseases
Abstract
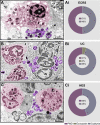
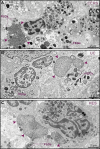
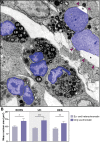
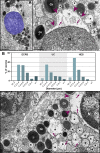
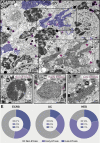
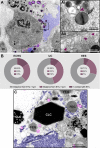
Eosinophilic diseases, also termed eosinophil-associated diseases (EADs), are characterized by eosinophil-rich inflammatory infiltrates and extensive eosinophil degranulation with clinically relevant organ pathology. Recent evidence shows that eosinophil cytolytic degranulation, that is, the release of intact, membrane-delimited granules that arises from the eosinophil cytolysis, occurs mainly through ETosis, meaning death with a cytolytic profile and extrusion of nucleus-originated DNA extracellular traps (ETs). The ultrastructural features of eosinophil ETosis (EETosis) have been studied mostly in vitro after stimulation, but are still poorly understood in vivo. Here, we investigated in detail, by transmission electron microscopy (TEM), the ultrastructure of EETosis in selected human EADs affecting several tissues and organ systems. Biopsies of patients diagnosed with eosinophilic chronic rhinosinusitis/ECRS (frontal sinus), ulcerative colitis/UC (intestine), and hypereosinophilic syndrome/HES (skin) were processed for conventional TEM. First, we found that a large proportion of tissue-infiltrated eosinophils in all diseases (~45-65% of all eosinophils) were undergoing cytolysis with release of free extracellular granules (FEGs). Second, we compared the morphology of tissue inflammatory eosinophils with that shown by in vitro ETosis-stimulated eosinophils. By applying single-cell imaging analysis, we sought typical early and late EETosis events: chromatin decondensation; nuclear delobulation and rounding; expanded nuclear area; nuclear envelope alterations and disruption; and extracellular decondensed chromatin spread as ETs. We detected that 53% (ECRS), 37% (UC), and 82% (HES) of all tissue cytolytic eosinophils had ultrastructural features of ETosis in different degrees. Eosinophils in early ETosis significantly increased their nuclear area compared to non-cytolytic eosinophils due to excessive chromatin decondensation and expansion observed before nuclear envelope disruption. ETosis led not only to the deposition of intact granules, but also to the release of eosinophil sombrero vesicles (EoSVs) and Charcot-Leyden crystals (CLCs). Free intact EoSVs and CLCs were associated with FEGs and extracellular DNA nets. Interestingly, not all cytolytic eosinophils in the same microenvironment exhibited ultrastructure of ETosis, thus indicating that different populations of eosinophils might be selectively activated into this pathway. Altogether, our findings captured an ultrastructural signature of EETosis in vivo in prototypic EADs highlighting the importance of this event as a form of eosinophil degranulation and release of inflammatory markers (EoSVs and CLCs).
Keywords: Charcot-Leyden crystals; eosinophil; eosinophil extracellular traps; eosinophil-associated diseases; eosinophilic chronic rhinosinusitis; hypereosinophilic syndrome; transmission electron microscopy; ulcerative colitis.
Copyright © 2022 Neves, Palazzi, Bonjour, Ueki, Weller and Melo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Extracellular sombrero vesicles are hallmarks of eosinophilic cytolytic degranulation in tissue sites of human diseases.J Leukoc Biol. 2024 Jul 25;116(2):398-408. doi: 10.1093/jleuko/qiae079. J Leukoc Biol. 2024. PMID: 38527801 Free PMC article.
-
Eosinophil ETosis-Mediated Release of Galectin-10 in Eosinophilic Granulomatosis With Polyangiitis.Arthritis Rheumatol. 2021 Sep;73(9):1683-1693. doi: 10.1002/art.41727. Epub 2021 Aug 11. Arthritis Rheumatol. 2021. PMID: 33750029 Free PMC article.
-
Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans.Blood. 2013 Mar 14;121(11):2074-83. doi: 10.1182/blood-2012-05-432088. Epub 2013 Jan 9. Blood. 2013. PMID: 23303825 Free PMC article.
-
How to detect eosinophil ETosis (EETosis) and extracellular traps.Allergol Int. 2021 Jan;70(1):19-29. doi: 10.1016/j.alit.2020.10.002. Epub 2020 Nov 12. Allergol Int. 2021. PMID: 33189567 Free PMC article. Review.
-
Eosinophil ETosis and DNA Traps: a New Look at Eosinophilic Inflammation.Curr Allergy Asthma Rep. 2016 Jul;16(8):54. doi: 10.1007/s11882-016-0634-5. Curr Allergy Asthma Rep. 2016. PMID: 27393701 Free PMC article. Review.
Cited by
-
The role of extracellular traps released by neutrophils, eosinophils, and macrophages in asthma.Respir Res. 2024 Jul 30;25(1):290. doi: 10.1186/s12931-024-02923-x. Respir Res. 2024. PMID: 39080638 Free PMC article. Review.
-
Extracellular sombrero vesicles are hallmarks of eosinophilic cytolytic degranulation in tissue sites of human diseases.J Leukoc Biol. 2024 Jul 25;116(2):398-408. doi: 10.1093/jleuko/qiae079. J Leukoc Biol. 2024. PMID: 38527801 Free PMC article.
-
Eosinophil extracellular traps in respiratory ailment: Pathogenic mechanisms and clinical translation.World J Otorhinolaryngol Head Neck Surg. 2023 Nov 1;10(3):213-224. doi: 10.1002/wjo2.138. eCollection 2024 Sep. World J Otorhinolaryngol Head Neck Surg. 2023. PMID: 39233861 Free PMC article. Review.
-
Eosinophil Peroxidase: A Biomarker for Eosinophilic Chronic Rhinosinusitis Agnostic of Polyp Status.Laryngoscope. 2024 Jan;134(1):69-78. doi: 10.1002/lary.30787. Epub 2023 May 31. Laryngoscope. 2024. PMID: 37255054 Free PMC article.
-
Eosinophil extracellular vesicles and DNA traps in allergic inflammation.Front Allergy. 2024 Aug 1;5:1448007. doi: 10.3389/falgy.2024.1448007. eCollection 2024. Front Allergy. 2024. PMID: 39148911 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

