Crosstalk between the B7/CD28 and EGFR pathways: Mechanisms and therapeutic opportunities
- PMID: 35873032
- PMCID: PMC9293717
- DOI: 10.1016/j.gendis.2021.08.009
Crosstalk between the B7/CD28 and EGFR pathways: Mechanisms and therapeutic opportunities
Abstract
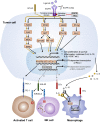
Somatic activating mutations in the epidermal growth factor receptor (EGFR) are one of the most common oncogenic drivers in cancers such as non-small-cell lung cancer (NSCLC), metastatic colorectal cancer, glioblastoma, head and neck cancer, pancreatic cancer, and breast cancer. Molecular-targeted agents against EGFR signaling pathways have shown robust clinical efficacy, but patients inevitably experience acquired resistance. Although immune checkpoint inhibitors (ICIs) targeting PD-1/PD-L1 have exhibited durable anti-tumor responses in a subset of patients across multiple cancer types, their efficacy is limited in cancers harboring activating gene alterations of EGFR. Increasing studies have demonstrated that upregulation of new B7/CD28 family members such as B7-H3, B7x and HHLA2, is associated with EGFR signaling and may contribute to resistance to EGFR-targeted therapies by creating an immunosuppressive tumor microenvironment (TME). In this review, we discuss the regulatory effect of EGFR signaling on the PD-1/PD-L1 pathway and new B7/CD28 family member pathways. Understanding these interactions may inform combination therapeutic strategies and potentially overcome the current challenge of resistance to EGFR-targeted therapies. We also summarize clinical data of anti-PD-1/PD-L1 therapies in EGFR-mutated cancers, as well as ongoing clinical trials of combination of EGFR-targeted therapies and anti-PD-1/PD-L1 immunotherapies.
Keywords: Combination therapies; EGFR; Immune checkpoint blockade; New B7/CD28 members; PD-1/PD-L1 pathway.
© 2021 Chongqing Medical University. Production and hosting by Elsevier B.V.
Figures
Similar articles
-
Epidermal Growth Factor Receptor (EGFR) Pathway, Yes-Associated Protein (YAP) and the Regulation of Programmed Death-Ligand 1 (PD-L1) in Non-Small Cell Lung Cancer (NSCLC).Int J Mol Sci. 2019 Aug 5;20(15):3821. doi: 10.3390/ijms20153821. Int J Mol Sci. 2019. PMID: 31387256 Free PMC article. Review.
-
Recent advancements in the B7/CD28 immune checkpoint families: new biology and clinical therapeutic strategies.Cell Mol Immunol. 2023 Jul;20(7):694-713. doi: 10.1038/s41423-023-01019-8. Epub 2023 Apr 17. Cell Mol Immunol. 2023. PMID: 37069229 Free PMC article. Review.
-
ILT4 inhibition prevents TAM- and dysfunctional T cell-mediated immunosuppression and enhances the efficacy of anti-PD-L1 therapy in NSCLC with EGFR activation.Theranostics. 2021 Jan 19;11(7):3392-3416. doi: 10.7150/thno.52435. eCollection 2021. Theranostics. 2021. PMID: 33537094 Free PMC article.
-
Immunotherapy-based combination strategies for treatment of EGFR-TKI-resistant non-small-cell lung cancer.Future Oncol. 2022 May;18(14):1757-1775. doi: 10.2217/fon-2021-0862. Epub 2022 Mar 2. Future Oncol. 2022. PMID: 35232247 Review.
-
PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions.Oncologist. 2019 Feb;24(Suppl 1):S31-S41. doi: 10.1634/theoncologist.2019-IO-S1-s05. Oncologist. 2019. PMID: 30819829 Free PMC article. Review.
Cited by
-
T cell senescence: a new perspective on immunotherapy in lung cancer.Front Immunol. 2024 Feb 13;15:1338680. doi: 10.3389/fimmu.2024.1338680. eCollection 2024. Front Immunol. 2024. PMID: 38415245 Free PMC article. Review.
-
Genetic alterations shape innate immune cells to foster immunosuppression and cancer immunotherapy resistance.Clin Exp Med. 2023 Dec;23(8):4289-4296. doi: 10.1007/s10238-023-01240-9. Epub 2023 Nov 1. Clin Exp Med. 2023. PMID: 37910258 Review.
-
Single-cell transcriptomic sequencing data reveal aberrant DNA methylation in SMAD3 promoter region in tumor-associated fibroblasts affecting molecular mechanism of radiosensitivity in non-small cell lung cancer.J Transl Med. 2024 Mar 16;22(1):288. doi: 10.1186/s12967-024-05057-2. J Transl Med. 2024. PMID: 38493128 Free PMC article.
-
Does Elevated Pre-Treatment Plasma PD-L1 Level Indicate an Increased Tumor Burden and Worse Prognosis in Metastatic Colorectal Cancer?J Clin Med. 2022 Aug 17;11(16):4815. doi: 10.3390/jcm11164815. J Clin Med. 2022. PMID: 36013050 Free PMC article.
-
Regulation of HHLA2 expression in kidney cancer and myeloid cells.BMC Cancer. 2023 Oct 27;23(1):1039. doi: 10.1186/s12885-023-11496-9. BMC Cancer. 2023. PMID: 37891555 Free PMC article.
References
-
- Bazley L.A., Gullick W.J. The epidermal growth factor receptor family. Endocr Relat Cancer. 2005;12(Suppl 1):S17–S27. - PubMed
-
- Cataldo V.D., Gibbons D.L., Pérez-Soler R., Quintás-Cardama A. Treatment of non-small-cell lung cancer with erlotinib or gefitinib. N Engl J Med. 2011;364(10):947–955. - PubMed
-
- Ciardiello F., Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358(11):1160–1174. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

