Stimulation of synaptic activity promotes TFEB-mediated clearance of pathological MAPT/Tau in cellular and mouse models of tauopathies
- PMID: 35867714
- PMCID: PMC9851246
- DOI: 10.1080/15548627.2022.2095791
Stimulation of synaptic activity promotes TFEB-mediated clearance of pathological MAPT/Tau in cellular and mouse models of tauopathies
Abstract
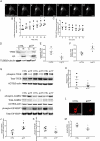
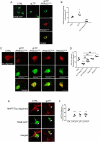
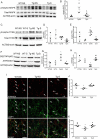
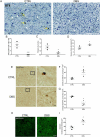
Synapses represent an important target of Alzheimer disease (AD), and alterations of their excitability are among the earliest changes associated with AD development. Synaptic activation has been shown to be protective in models of AD, and deep brain stimulation (DBS), a surgical strategy that modulates neuronal activity to treat neurological and psychiatric disorders, produced positive effects in AD patients. However, the molecular mechanisms underlying the protective role(s) of brain stimulation are still elusive. We have previously demonstrated that induction of synaptic activity exerts protection in mouse models of AD and frontotemporal dementia (FTD) by enhancing the macroautophagy/autophagy flux and lysosomal degradation of pathological MAPT/Tau. We now provide evidence that TFEB (transcription factor EB), a master regulator of lysosomal biogenesis and autophagy, is a key mediator of this cellular response. In cultured primary neurons from FTD-transgenic mice, synaptic stimulation inhibits MTORC1 signaling, thus promoting nuclear translocation of TFEB, which, in turn, induces clearance of MAPT/Tau oligomers. Conversely, synaptic activation fails to promote clearance of toxic MAPT/Tau in neurons expressing constitutively active RRAG GTPases, which sequester TFEB in the cytosol, or upon TFEB depletion. Activation of TFEB is also confirmed in vivo in DBS-stimulated AD mice. We also demonstrate that DBS reduces pathological MAPT/Tau and promotes neuroprotection in Parkinson disease patients with tauopathy. Altogether our findings indicate that stimulation of synaptic activity promotes TFEB-mediated clearance of pathological MAPT/Tau. This mechanism, underlying the protective effect of DBS, provides encouraging support for the use of synaptic stimulation as a therapeutic treatment against tauopathies.Abbreviations: 3xTg-AD: triple transgenic AD mice; AD: Alzheimer disease; CSA: cyclosporine A; DBS: deep brain stimulation; DIV: days in vitro; EC: entorhinal cortex; FTD: frontotemporal dementia; gLTP: glycine-induced long-term potentiation; GPi: internal segment of the globus pallidus; PD: Parkinson disease; STN: subthalamic nucleus; TFEB: transcription factor EB.
Keywords: Alzheimer; autophagy; deep brain stimulation; lysosome; neuron; synapse; tau.
Conflict of interest statement
Authors declare that the research was conducted in the absence of any potential conflict of interest.
Figures

Similar articles
-
Celastrol, a TFEB (transcription factor EB) agonist, is a promising drug candidate for Alzheimer disease.Autophagy. 2022 Jul;18(7):1740-1742. doi: 10.1080/15548627.2022.2046437. Epub 2022 Mar 6. Autophagy. 2022. PMID: 35253615 Free PMC article.
-
Synaptic activity protects against AD and FTD-like pathology via autophagic-lysosomal degradation.Mol Psychiatry. 2018 Jun;23(6):1530-1540. doi: 10.1038/mp.2017.142. Epub 2017 Jul 11. Mol Psychiatry. 2018. PMID: 28696431 Free PMC article.
-
Selective clearance of aberrant tau proteins and rescue of neurotoxicity by transcription factor EB.EMBO Mol Med. 2014 Sep;6(9):1142-60. doi: 10.15252/emmm.201303671. EMBO Mol Med. 2014. PMID: 25069841 Free PMC article.
-
TFEB in Alzheimer's disease: From molecular mechanisms to therapeutic implications.Neurobiol Dis. 2022 Oct 15;173:105855. doi: 10.1016/j.nbd.2022.105855. Epub 2022 Aug 27. Neurobiol Dis. 2022. PMID: 36031168 Review.
-
The dual and emerging role of physical exercise-induced TFEB activation in the protection against Alzheimer's disease.J Cell Physiol. 2023 May;238(5):954-965. doi: 10.1002/jcp.31005. Epub 2023 Apr 3. J Cell Physiol. 2023. PMID: 37013375 Review.
Cited by
-
Effect of Subthalamic Stimulation and Electrode Implantation in the Striatal Microenvironment in a Parkinson's Disease Rat Model.Int J Mol Sci. 2022 Oct 11;23(20):12116. doi: 10.3390/ijms232012116. Int J Mol Sci. 2022. PMID: 36292973 Free PMC article.
-
Systematic review of rodent studies of deep brain stimulation for the treatment of neurological, developmental and neuropsychiatric disorders.Transl Psychiatry. 2024 Apr 11;14(1):186. doi: 10.1038/s41398-023-02727-5. Transl Psychiatry. 2024. PMID: 38605027 Free PMC article.
-
Activity-dependent mechanisms of neuroprotection: promising avenues against dementia.Neural Regen Res. 2024 Jul 1;19(7):1409-1410. doi: 10.4103/1673-5374.387985. Epub 2023 Nov 8. Neural Regen Res. 2024. PMID: 38051871 Free PMC article. No abstract available.
-
The synthetic TRPML1 agonist ML-SA1 rescues Alzheimer-related alterations of the endosomal-autophagic-lysosomal system.J Cell Sci. 2023 Mar 15;136(6):jcs259875. doi: 10.1242/jcs.259875. Epub 2023 Mar 21. J Cell Sci. 2023. PMID: 36825945 Free PMC article.
-
Truncated tau interferes with the autophagy and endolysosomal pathway and results in lipid accumulation.Cell Mol Life Sci. 2024 Jul 15;81(1):304. doi: 10.1007/s00018-024-05337-6. Cell Mol Life Sci. 2024. PMID: 39009859 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
