Biological fate and interaction with cytochromes P450 of the nanocarrier material, d- α-tocopheryl polyethylene glycol 1000 succinate
- PMID: 35865103
- PMCID: PMC9293673
- DOI: 10.1016/j.apsb.2022.01.014
Biological fate and interaction with cytochromes P450 of the nanocarrier material, d- α-tocopheryl polyethylene glycol 1000 succinate
Abstract
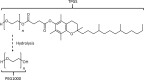
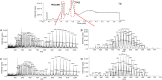
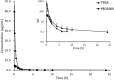
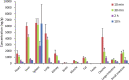
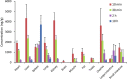
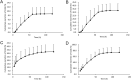
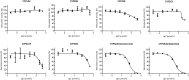
d-α-Tocopheryl polyethylene glycol 1000 succinate (TPGS, also known as vitamin E-TPGS) is a biodegradable amphiphilic polymer prepared by esterification of vitamin E with polyethylene glycol (PEG) 1000. It is approved by the US Food and Drug Administration (FDA) and has found wide application in nanocarrier drug delivery systems (NDDS). Fully characterizing the in vivo fate and pharmacokinetic behavior of TPGS is important to promote the further development of TPGS-based NDDS. However, to date, a bioassay for the simultaneous quantitation of TPGS and its metabolite, PEG1000, has not been reported. In the present study, we developed such an innovative bioassay and used it to investigate the pharmacokinetics, tissue distribution and excretion of TPGS and PEG1000 in rat after oral and intravenous dosing. In addition, we evaluated the interaction of TPGS with cytochromes P450 (CYP450s) in human liver microsomes. The results show that TPGS is poorly absorbed after oral administration with very low bioavailability and that, after intravenous administration, TPGS and PEG1000 are mainly distributed to the spleen, liver, lung and kidney before both being slowly eliminated in urine and feces as PEG1000. In vitro studies show the inhibition of human CYP450 enzymes by TPGS is limited to a weak inhibition of CYP3A4. Overall, our results provide a clear picture of the in vivo fate of TPGS which will be useful in evaluating the safety of TPGS-based NDDS in clinical use and in promoting their further development.
Keywords: Cytochrome P450; Excretion; LC‒MS/MS; Metabolism; Nanocarrier materials; Pharmacokinetics; TPGS; Tissue distribution.
© 2022 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


Similar articles
-
The biological fate of the polymer nanocarrier material monomethoxy poly(ethylene glycol)-block-poly(d,l-lactic acid) in rat.Acta Pharm Sin B. 2021 Apr;11(4):1003-1009. doi: 10.1016/j.apsb.2021.02.018. Epub 2021 Mar 4. Acta Pharm Sin B. 2021. PMID: 33996412 Free PMC article.
-
D-α-tocopheryl polyethylene glycol 1000 succinate-containing vehicles provide no detectable chemoprotection from oxidative damage.J Appl Toxicol. 2015 Jul;35(7):791-8. doi: 10.1002/jat.3072. Epub 2014 Oct 28. J Appl Toxicol. 2015. PMID: 25348750
-
Enhanced water solubility, antioxidant activity, and oral absorption of hesperetin by D-α-tocopheryl polyethylene glycol 1000 succinate and phosphatidylcholine.J Zhejiang Univ Sci B. 2019 Mar.;20(3):273-281. doi: 10.1631/jzus.B1800346. J Zhejiang Univ Sci B. 2019. PMID: 30829014 Free PMC article.
-
Recent developments in d-α-tocopheryl polyethylene glycol-succinate-based nanomedicine for cancer therapy.Drug Deliv. 2017 Nov;24(1):1831-1842. doi: 10.1080/10717544.2017.1406561. Drug Deliv. 2017. PMID: 29182031 Free PMC article. Review.
-
The applications of Vitamin E TPGS in drug delivery.Eur J Pharm Sci. 2013 May 13;49(2):175-86. doi: 10.1016/j.ejps.2013.02.006. Epub 2013 Feb 26. Eur J Pharm Sci. 2013. PMID: 23485439 Review.
Cited by
-
Neem Oil or Almond Oil Nanoemulsions for Vitamin E Delivery: From Structural Evaluation to in vivo Assessment of Antioxidant and Anti-Inflammatory Activity.Int J Nanomedicine. 2022 Dec 20;17:6447-6465. doi: 10.2147/IJN.S376750. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36573206 Free PMC article.
-
Differential Mobility Spectrometry-Tandem Mass Spectrometry with Multiple Ion Monitoring Coupled with in Source-Collision Induced Dissociation: A New Strategy for the Quantitative Analysis of Pharmaceutical Polymer Excipients in Rat Plasma.Molecules. 2023 Jun 15;28(12):4782. doi: 10.3390/molecules28124782. Molecules. 2023. PMID: 37375337 Free PMC article.
-
Full-profile pharmacokinetics, anticancer activity and toxicity of an extended release trivalent PEGylated irinotecan prodrug.Acta Pharm Sin B. 2023 Aug;13(8):3444-3453. doi: 10.1016/j.apsb.2023.01.011. Epub 2023 Jan 10. Acta Pharm Sin B. 2023. PMID: 37655324 Free PMC article.
References
-
- Guo Y.Y., Luo J., Tan S.W., Otieno B.O., Zhang Z.P. The applications of Vitamin E TPGS in drug delivery. Eur J Pharmaceut Sci. 2013;49:175–186. - PubMed
-
- Zhang Z.P., Tan S.W., Feng S.S. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 2012;33:4889–4906. - PubMed
-
- Collnot E.M., Baldes C., Wempe M.F., Kappl R., Huttermann J., Hyatt J.A., et al. Mechanism of inhibition of P-glycoprotein mediated efflux by vitamin E TPGS: influence on ATPase activity and membrane fluidity. Mol Pharm. 2007;4:465–474. - PubMed
LinkOut - more resources
Full Text Sources

