LHX2 facilitates the progression of nasopharyngeal carcinoma via activation of the FGF1/FGFR axis
- PMID: 35864158
- PMCID: PMC9519904
- DOI: 10.1038/s41416-022-01902-7
LHX2 facilitates the progression of nasopharyngeal carcinoma via activation of the FGF1/FGFR axis
Abstract
Background: Distant metastasis and recurrence remain the main obstacle to nasopharyngeal carcinoma (NPC) treatment. However, the molecular mechanisms underlying NPC growth and metastasis are poorly understood.
Methods: LHX2 expression was examined in NPC cell lines and NPC tissues using quantitative reverse transcription-polymerase chain reaction, western blotting and Immunohistochemistry assay. NPC cells overexpressing or silencing LHX2 were used to perform CCK-8 assay, colony-formation assay, EdU assay, wound-healing and invasion assays in vitro. Xenograft tumour models and lung metastasis models were involved for the in vivo assays. The Gene Set Enrichment Analysis (GSEA), ELISA assay, western blot, chromatin immunoprecipitation (ChIP) assay and Luciferase reporter assay were applied for the downstream target mechanism investigation.
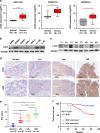
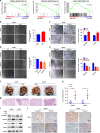
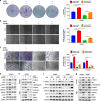
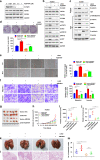
Results: LIM-homeodomain transcription factor 2 (LHX2) was upregulated in NPC tissues and cell lines. Elevated LHX2 was closely associated with poor survival in NPC patients. Ectopic LHX2 overexpression dramatically promoted the growth, migration and invasion of NPC cells both in vitro and in vivo. Mechanistically, LHX2 transcriptionally increased the fibroblast growth factor 1 (FGF1) expression, which in turn activated the phosphorylation of STAT3 (signal transducer and activator of transcription 3), ERK1/2 (extracellular regulated protein kinases 1/2) and AKT signalling pathways in an autocrine and paracrine manner, thereby promoting the growth and metastasis of NPC. Inhibition of FGF1 with siRNA or FGFR inhibitor blocked LHX2-induced nasopharyngeal carcinoma cell growth, migration and invasion.
Conclusions: Our study identifies the LHX2-FGF1-FGFR axis plays a key role in NPC progression and provides a potential target for NPC therapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
MicroRNA-506 inhibits tumor growth and metastasis in nasopharyngeal carcinoma through the inactivation of the Wnt/β-catenin signaling pathway by down-regulating LHX2.J Exp Clin Cancer Res. 2019 Feb 21;38(1):97. doi: 10.1186/s13046-019-1023-4. J Exp Clin Cancer Res. 2019. Retraction in: J Exp Clin Cancer Res. 2021 Sep 30;40(1):309. doi: 10.1186/s13046-021-02113-3 PMID: 30791932 Free PMC article. Retracted. Clinical Trial.
-
Long non-coding RNA PTPRG-AS1/microRNA-124-3p regulates radiosensitivity of nasopharyngeal carcinoma via the LIM Homeobox 2-dependent Notch pathway through competitive endogenous RNA mechanism.Bioengineered. 2022 Apr;13(4):8208-8225. doi: 10.1080/21655979.2022.2037364. Bioengineered. 2022. PMID: 35300558 Free PMC article.
-
Function of AXL and molecular mechanisms in regulation of nasopharyngeal carcinoma.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022 Jun 28;47(6):685-697. doi: 10.11817/j.issn.1672-7347.2022.210786. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 35837768 Free PMC article. Chinese, English.
-
Circ_0000523 regulates miR-1184/COL1A1/PI3K/Akt pathway to promote nasopharyngeal carcinoma progression.Apoptosis. 2022 Oct;27(9-10):751-761. doi: 10.1007/s10495-022-01743-y. Epub 2022 Jun 27. Apoptosis. 2022. PMID: 35759163
-
Activation of miR-21 by STAT3 induces proliferation and suppresses apoptosis in nasopharyngeal carcinoma by targeting PTEN gene.PLoS One. 2014 Nov 3;9(11):e109929. doi: 10.1371/journal.pone.0109929. eCollection 2014. PLoS One. 2014. PMID: 25365510 Free PMC article.
Cited by
-
LHX2 Enhances the Malignant Phenotype of Esophageal Squamous Cell Carcinoma by Upregulating the Expression of SERPINE2.Genes (Basel). 2022 Aug 16;13(8):1457. doi: 10.3390/genes13081457. Genes (Basel). 2022. PMID: 36011368 Free PMC article.
-
Onco-Ontogeny of Squamous Cell Cancer of the First Pharyngeal Arch Derivatives.Int J Mol Sci. 2024 Sep 16;25(18):9979. doi: 10.3390/ijms25189979. Int J Mol Sci. 2024. PMID: 39337467 Free PMC article. Review.
-
LHX2 Is a Potential Biomarker and Associated with Immune Infiltration in Breast Cancer.Cancers (Basel). 2023 May 16;15(10):2773. doi: 10.3390/cancers15102773. Cancers (Basel). 2023. PMID: 37345110 Free PMC article.
References
-
- Li Y, Tang LQ, Liu LT, Guo SS, Liang YJ, Sun XS, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma in children and adolescents: a matched cohort analysis. Cancer Res Treat. 2018;50:1304–15. doi: 10.4143/crt.2017.463. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

