Extracellular Loops of the Treponema pallidum FadL Orthologs TP0856 and TP0858 Elicit IgG Antibodies and IgG+-Specific B-Cells in the Rabbit Model of Experimental Syphilis
- PMID: 35862766
- PMCID: PMC9426418
- DOI: 10.1128/mbio.01639-22
Extracellular Loops of the Treponema pallidum FadL Orthologs TP0856 and TP0858 Elicit IgG Antibodies and IgG+-Specific B-Cells in the Rabbit Model of Experimental Syphilis
Abstract
The resurgence of syphilis in the new millennium has called attention to the importance of a vaccine for global containment strategies. Studies with immune rabbit serum (IRS) indicate that a syphilis vaccine should elicit antibodies (Abs) that promote opsonophagocytosis of treponemes by activated macrophages. The availability of three-dimensional models for Treponema pallidum's (Tp) repertoire of outer membrane proteins (OMPs) provides an architectural framework for identification of candidate vaccinogens with extracellular loops (ECLs) as the targets for protective Abs. Herein, we used Pyrococcus furiosus thioredoxin (PfTrx) as a scaffold to display Tp OMP ECLs to interrogate sera and peripheral blood mononuclear cells (PBMCs) from immune rabbits for ECL-specific Abs and B cells. We validated this approach using a PfTrx scaffold presenting ECL4 from BamA, a known opsonic target. Using scaffolds displaying ECLs of the FadL orthologs TP0856 and TP0858, we determined that ECL2 and ECL4 of both proteins are strongly antigenic. Comparison of ELISA and immunoblot results suggested that the PfTrx scaffolds present conformational and linear epitopes. We then used the FadL ECL2 and ECL4 PfTrx constructs as "hooks" to confirm the presence of ECL-specific B cells in PBMCs from immune rabbits. Our results pinpoint immunogenic ECLs of two newly discovered OMPs, while advancing the utility of the rabbit model for circumventing bottlenecks in vaccine development associated with large-scale production of folded OMPs. They also lay the groundwork for production of rabbit monoclonal Abs (MAbs) to characterize potentially protective ECL epitopes at the atomic level. IMPORTANCE Recent identification and structural modeling of Treponema pallidum's (Tp) repertoire of outer membrane proteins (OMPs) represent a critical breakthrough in the decades long quest for a syphilis vaccine. However, little is known about the antigenic nature of these β-barrel-forming OMPs and, more specifically, their surface exposed regions, the extracellular loops (ECLs). In this study, using Pyrococcus furiosus thioredoxin (PfTrx) as a scaffold to display Tp OMP ECLs, we interrogated immune rabbit sera and peripheral blood mononuclear cells for the presence of antibodies (Abs) and circulating rare antigen-specific B cells. Our results pinpoint immunogenic ECLs of two newly discovered OMPs, while advancing the utility of the rabbit model for surveying the entire Tp OMPeome for promising OMP vaccinogens. This work represents a major advancement toward characterizing potentially protective OMP ECLs and future vaccine studies. Additionally, this strategy could be applied to OMPs of nonspirochetal bacterial pathogens.
Keywords: B cells; FadL; Treponema pallidum; extracellular loop; outer membrane protein; syphilis; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
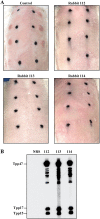





References
-
- Gottlieb SL, Deal CD, Giersing B, Rees H, Bolan G, Johnston C, Timms P, Gray-Owen SD, Jerse AE, Cameron CE, Moorthy VS, Kiarie J, Broutet N. 2016. The global roadmap for advancing development of vaccines against sexually transmitted infections: update and next steps. Vaccine 34:2939–2947. doi:10.1016/j.vaccine.2016.03.111. - DOI - PMC - PubMed
-
- Luthra A, Montezuma-Rusca JM, La Vake CJ, LeDoyt M, Delgado KN, Davenport TC, Fiel-Gan M, Caimano MJ, Radolf JD, Hawley KL. 2020. Evidence that immunization with TP0751, a bipartite Treponema pallidum lipoprotein with an intrinsically disordered region and lipocalin fold, fails to protect in the rabbit model of experimental syphilis. PLoS Pathog 16:e1008871. doi:10.1371/journal.ppat.1008871. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
