Costs and cost-effectiveness of HIV early infant diagnosis in low- and middle-income countries: a scoping review
- PMID: 35841117
- PMCID: PMC9284833
- DOI: 10.1186/s40249-022-01006-7
Costs and cost-effectiveness of HIV early infant diagnosis in low- and middle-income countries: a scoping review
Abstract
Background: Continuing progress in the global pediatric human immunodeficiency virus (HIV) response depends on timely identification and care of infants with HIV. As countries scale-out improvements to HIV early infant diagnosis (EID), economic evaluations are needed to inform program design and implementation. This scoping review aimed to summarize the available evidence and discuss practical implications of cost and cost-effectiveness analyses of HIV EID.
Methods: We systematically searched bibliographic databases (Embase, MEDLINE and EconLit) and grey literature for economic analyses of HIV EID in low- and middle-income countries published between January 2008 and June 2021. We extracted data on unit costs, cost savings, and incremental cost-effectiveness ratios as well as outcomes related to health and the HIV EID care process and summarized results in narrative and tabular formats. We converted unit costs to 2021 USD for easier comparison of costs across studies.
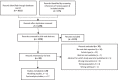
Results: After title and abstract screening of 1278 records and full-text review of 99 records, we included 29 studies: 17 cost analyses and 12 model-based cost-effectiveness analyses. Unit costs were 21.46-51.80 USD for point-of-care EID tests and 16.21-42.73 USD for laboratory-based EID tests. All cost-effectiveness analyses stated at least one of the interventions evaluated to be cost-effective. Most studies reported costs of EID testing strategies; however, few studies assessed the same intervention or reported costs in the same way, making comparison of costs across studies challenging. Limited data availability of context-appropriate costs and outcomes of children with HIV as well as structural heterogeneity of cost-effectiveness modelling studies limits generalizability of economic analyses of HIV EID.
Conclusions: The available cost and cost-effectiveness evidence for EID of HIV, while not directly comparable across studies, covers a broad range of interventions and suggests most interventions designed to improve EID are cost-effective or cost-saving. Further studies capturing costs and benefits of EID services as they are delivered in real-world settings are needed.
Keywords: Cost effectiveness; Diagnostics; Early infant diagnosis; Health systems; Low- and middle-income countries; Point of care.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Similar articles
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Strategies used for childhood chronic functional constipation: the SUCCESS evidence synthesis.Health Technol Assess. 2024 Jan;28(5):1-266. doi: 10.3310/PLTR9622. Health Technol Assess. 2024. PMID: 38343084 Free PMC article.
-
Comprehensive care programmes for children with medical complexity.Cochrane Database Syst Rev. 2024 May 30;5(5):CD013329. doi: 10.1002/14651858.CD013329.pub2. Cochrane Database Syst Rev. 2024. PMID: 38813833 Review.
-
School-based education programmes for the prevention of unintentional injuries in children and young people.Cochrane Database Syst Rev. 2016 Dec 27;12(12):CD010246. doi: 10.1002/14651858.CD010246.pub2. Cochrane Database Syst Rev. 2016. PMID: 28026877 Free PMC article. Review.
-
Genedrive kit for detecting single nucleotide polymorphism m.1555A>G in neonates and their mothers: a systematic review and cost-effectiveness analysis.Health Technol Assess. 2024 Oct;28(75):1-75. doi: 10.3310/TGAC4201. Health Technol Assess. 2024. PMID: 39487741 Free PMC article.
Cited by
-
Improving early infant diagnosis for HIV-exposed infants using unmanned aerial vehicles for blood sample transportation in Conakry, Guinea: a comparative cost-effectiveness analysis.BMJ Glob Health. 2023 Nov;8(11):e012522. doi: 10.1136/bmjgh-2023-012522. BMJ Glob Health. 2023. PMID: 37984898 Free PMC article.
-
Cost-effectiveness of point-of-care versus centralised, laboratory-based nucleic acid testing for diagnosis of HIV in infants: a systematic review of modelling studies.Lancet HIV. 2023 May;10(5):e320-e331. doi: 10.1016/S2352-3018(23)00029-2. Lancet HIV. 2023. PMID: 37149292 Free PMC article.
-
Increasing the initiation of antiretroviral therapy through optimal placement of diagnostic technologies for pediatric HIV in Zimbabwe: A modeling analysis.Int J Infect Dis. 2023 Sep;134:31-38. doi: 10.1016/j.ijid.2023.05.013. Epub 2023 May 16. Int J Infect Dis. 2023. PMID: 37196759 Free PMC article.
References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS). AIDSinfo data sheet: pregnant women needing ARV for PMTCT 2020. https://aidsinfo.unaids.org/. Accessed 1 Dec 2021.
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS). AIDSinfo data sheet: new HIV infections—children (0–14) 2020. https://aidsinfo.unaids.org/. Accessed 1 Dec 2021.
-
- World Health Organization. HIV diagnosis and ARV use in HIV-exposed infants: a programmatic update. 2018.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials