Immune checkpoint inhibitor (ICI)-based treatment beyond progression with prior immunotherapy in patients with stage IV non-small cell lung cancer: a retrospective study
- PMID: 35832458
- PMCID: PMC9271428
- DOI: 10.21037/tlcr-22-376
Immune checkpoint inhibitor (ICI)-based treatment beyond progression with prior immunotherapy in patients with stage IV non-small cell lung cancer: a retrospective study
Abstract
Background: Although immune checkpoint inhibitors (ICIs) provide unprecedented survival improvement for patients with advanced non-small cell lung cancer (NSCLC), disease progression inevitably occurs. After ICIs failure, limited data exist on whether ICI-based treatment beyond progression (TBP) may be beneficial to advanced NSCLC. This retrospective study aimed to evaluate the efficacy of this treatment approach in advanced NSCLC and identify potential beneficial factors.
Methods: Patients with stage IV NSCLC who received ICI-based treatment after the failure of prior PD-1/PD-L1 inhibitor treatments (monotherapy or combination therapy) between January 2016 and July 2020 were enrolled. Their clinical characteristics and treatment procedures were collected, and the follow-up would be performed.
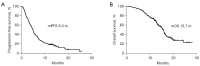
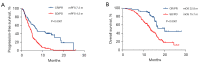
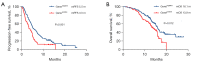
Results: A total of 204 patients were included. All patients had disease progression after prior immunotherapy, with 49.5% (101/204) of patients presenting with new metastasis lesions and the rest 50.5% (103/204) of patients' progression on originate lesions. Within the entire cohort, the median progression-free survival (PFS) and median overall survival (OS) of ICI-based TBP with prior immunotherapy were 5.0 months (95% CI: 4.5-5.5 months) and 15.7 months (95% CI: 14.7-16.8 months), respectively. The objective response rate (ORR) and disease control rate (DCR) were 9.3% and 74.0%, respectively. According to the multivariate analysis, ICI-based combination therapy [PFS: hazard ratio (HR), 0.48, 95% confidence interval (CI): 0.28-0.84, P=0.011] (OS: HR, 0.44, 95% CI: 0.23-0.85, P=0.014), not having targetable gene alterations (PFS: HR, 0.56, 95% CI: 0.40-0.79, P=0.001) (OS: HR, 0.57, 95% CI: 0.37-0.87, P=0.009), and good response to prior immunotherapy (PFS: HR, 0.36, 95% CI: 0.24-0.53, P<0.0001) (OS: HR, 0.31, 95% CI: 0.19-0.52, P<0.0001) were independently associated with improved PFS and OS. Moreover, disease progression due to appearances of new metastasis (OS: HR, 0.56, 95% CI: 0.37-0.84, P=0.005) was only associated with better OS.
Conclusions: While the ORR in patients with advanced NSCLC receiving ICI-based TBP with prior immunotherapy was limited, the DCR was relatively high in our study which is encouraging. ICI-based treatment strategy may be a reasonable option for patients who progressed from prior immunotherapy. Further prospective studies on larger sample size are warranted.
Keywords: Non-small cell lung cancer (NSCLC); immune checkpoint inhibitors (ICIs); immunotherapy; metastatic; treatment beyond prior immunotherapy.
2022 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-376/coif). TTS reports that he provides strategic and scientific recommendations as a member of the Advisory Board and speaker for Novocure, Inc., and also as a member of the Advisory Board to Galera Therapeutics, which are not in any way associated with the content or disease site as presented in this manuscript. The other authors have no conflicts of interest to declare.
Figures



Similar articles
-
[Real-world study on the efficacy and prognostic predictive biomarker of patients with metastatic non-small cell lung cancer treated with programmed death-1/programmed death ligand 1 inhibitors].Zhonghua Zhong Liu Za Zhi. 2022 May 23;44(5):416-424. doi: 10.3760/cma.j.cn112152-20210709-00504. Zhonghua Zhong Liu Za Zhi. 2022. PMID: 35615798 Chinese.
-
Immunotherapy for patients with advanced non-small cell lung cancer harboring oncogenic driver alterations other than EGFR: a multicenter real-world analysis.Transl Lung Cancer Res. 2024 Apr 29;13(4):861-874. doi: 10.21037/tlcr-24-116. Epub 2024 Apr 24. Transl Lung Cancer Res. 2024. PMID: 38736501 Free PMC article.
-
The efficacy and safety of immune checkpoint inhibitors combined with chemotherapy or anti-angiogenic therapy as a second-line or later treatment option for advanced non-small cell lung cancer: a retrospective comparative cohort study.Transl Lung Cancer Res. 2022 Oct;11(10):2111-2124. doi: 10.21037/tlcr-22-697. Transl Lung Cancer Res. 2022. PMID: 36386462 Free PMC article.
-
Efficacy of PD-1/PD-L1 Inhibitors versus Chemotherapy in Lung Cancer with Brain Metastases: A Systematic Review and Meta-Analysis.J Immunol Res. 2022 May 20;2022:4518898. doi: 10.1155/2022/4518898. eCollection 2022. J Immunol Res. 2022. PMID: 35637793 Free PMC article. Review.
-
Efficacy of NSCLC Rechallenge with Immune Checkpoint Inhibitors following Disease Progression or Relapse.Cancers (Basel). 2024 Mar 18;16(6):1196. doi: 10.3390/cancers16061196. Cancers (Basel). 2024. PMID: 38539530 Free PMC article. Review.
Cited by
-
The prognostic factors of clinical outcomes in non-small cell lung cancer patients receiving subsequent treatments after progression on initial immunotherapy.J Thorac Dis. 2024 Sep 30;16(9):6012-6023. doi: 10.21037/jtd-24-57. Epub 2024 Sep 26. J Thorac Dis. 2024. PMID: 39444890 Free PMC article.
-
[Advances of Immunotherapy Resistance and Coping Strategies in Non-small Cell Lung Cancer].Zhongguo Fei Ai Za Zhi. 2023 Jan 20;26(1):66-77. doi: 10.3779/j.issn.1009-3419.2023.102.03. Zhongguo Fei Ai Za Zhi. 2023. PMID: 36792083 Free PMC article. Review. Chinese.
-
Genome-wide Analysis Identified SEMA4D, Novel Candidate Gene for Temperature Sensitivity in Patients With Non-Small Cell Lung Cancer.Integr Cancer Ther. 2024 Jan-Dec;23:15347354241233544. doi: 10.1177/15347354241233544. Integr Cancer Ther. 2024. PMID: 38469817 Free PMC article.
-
Research progress of biomarkers in the prediction of anti-PD-1/PD-L1 immunotherapeutic efficiency in lung cancer.Front Immunol. 2023 Jul 3;14:1227797. doi: 10.3389/fimmu.2023.1227797. eCollection 2023. Front Immunol. 2023. PMID: 37465684 Free PMC article. Review.
-
Future Perspectives in the Second Line Therapeutic Setting for Non-Oncogene Addicted Non-Small-Cell Lung Cancer.Cancers (Basel). 2023 Nov 21;15(23):5505. doi: 10.3390/cancers15235505. Cancers (Basel). 2023. PMID: 38067208 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
