Low Serum Dehydroepiandrosterone and Dehydroepiandrosterone Sulfate Are Associated With Coronary Heart Disease in Men With Type 2 Diabetes Mellitus
- PMID: 35832423
- PMCID: PMC9271610
- DOI: 10.3389/fendo.2022.890029
Low Serum Dehydroepiandrosterone and Dehydroepiandrosterone Sulfate Are Associated With Coronary Heart Disease in Men With Type 2 Diabetes Mellitus
Abstract
Aims: Sex hormones play an important role in the pathogenesis of cardiovascular disease (CVD). This cross-sectional study aimed to explore the associations of dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) with coronary heart disease (CHD) and stroke in middle-aged and elderly patients with type 2 diabetes mellitus (T2DM).
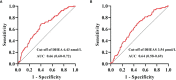
Materials and methods: A total of 995 patients with T2DM were included in the study analysis. Serum levels of DHEA and DHEAS were quantified using liquid chromatography-tandem mass spectrometry. Binary logistic regression analyses were performed to assess the associations of DHEA and DHEAS with CHD and stroke. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal DHEA and DHEAS cutoff values for the detection of CHD in men with T2DM.
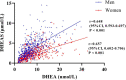
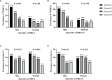
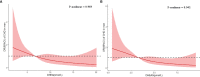
Results: In men with T2DM, after adjustment for potential confounders in model 3, the risk of CHD decreased with an increasing serum DHEA level [odds ratio (OR) = 0.38, quartile 4 vs. quartile 1; 95% confidence interval (CI) = 0.16-0.90; p = 0.037 for trend). Consistently, when considered as a continuous variable, this association remained significant in the fully adjusted model (OR = 0.59, 95% CI = 0.40-0.87, p < 0.05). When taken as a continuous variable in model 3, serum DHEAS level was also inversely related to the risk of CHD among men (OR = 0.56, 95% CI = 0.38-0.82, p < 0.05). Similarly, this relationship remained statistically significant when DHEAS was categorized into quartiles (OR = 0.27, quartile 4 vs. quartile 1; 95% CI = 0.11-0.67; p = 0.018 for trend). ROC curve analyses revealed that the optimal cutoff values to detect CHD in men with T2DM were 6.43 nmol/L for DHEA and 3.54 μmol/L for DHEAS. In contrast, no significant associations were found between DHEA and DHEAS on the one hand and stroke on the other in men and women with T2DM (all p > 0.05).
Conclusions: Serum DHEA and DHEAS were significantly and negatively associated with CHD in middle-aged and elderly men with T2DM. This study suggests potential roles of DHEA and DHEAS in CHD pathogenesis.
Keywords: coronary heart disease; dehydroepiandrosterone; dehydroepiandrosterone sulfate; stroke; type 2 diabetes mellitus.
Copyright © 2022 Zhang, Xiao, Liu, He, Cui, Tang, Li and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Low Serum Dehydroepiandrosterone Is Associated With Diabetic Kidney Disease in Men With Type 2 Diabetes Mellitus.Front Endocrinol (Lausanne). 2022 Jun 15;13:915494. doi: 10.3389/fendo.2022.915494. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35784547 Free PMC article.
-
Sex-specific association of serum dehydroepiandrosterone and its sulfate levels with osteoporosis in type 2 diabetes.J Bone Miner Metab. 2024 May;42(3):361-371. doi: 10.1007/s00774-024-01511-9. Epub 2024 May 20. J Bone Miner Metab. 2024. PMID: 38769209
-
Serum dehydroepiandrosterone levels are associated with lower risk of type 2 diabetes: the Rotterdam Study.Diabetologia. 2017 Jan;60(1):98-106. doi: 10.1007/s00125-016-4136-8. Epub 2016 Oct 22. Diabetologia. 2017. PMID: 27771738 Free PMC article.
-
Do DHEA/DHEAS play a protective role in coronary heart disease?Physiol Res. 2000;49 Suppl 1:S43-56. Physiol Res. 2000. PMID: 10984071 Review.
-
Dehydroepiandrosterone, dehydroepiandrosterone sulphate and cardiovascular disease.J Endocrinol. 1996 Sep;150 Suppl:S149-53. J Endocrinol. 1996. PMID: 8943798 Review.
Cited by
-
Low serum dehydroepiandrosterone is associated with diabetic dyslipidemia risk in males with type 2 diabetes.Front Endocrinol (Lausanne). 2023 Nov 24;14:1272797. doi: 10.3389/fendo.2023.1272797. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38075062 Free PMC article.
-
Low serum dehydroepiandrosterone levels are associated with diabetic retinopathy in patients with type 2 diabetes mellitus.J Diabetes Investig. 2023 May;14(5):675-685. doi: 10.1111/jdi.13997. Epub 2023 Feb 22. J Diabetes Investig. 2023. PMID: 36811237 Free PMC article.
-
Diabetes Mellitus Should Be Considered While Analysing Sarcopenia-Related Biomarkers.J Clin Med. 2024 Feb 15;13(4):1107. doi: 10.3390/jcm13041107. J Clin Med. 2024. PMID: 38398421 Free PMC article. Review.
-
Effects of steroid hormones on lipid metabolism in sexual dimorphism: A Mendelian randomization study.Front Endocrinol (Lausanne). 2023 Jan 16;13:1119154. doi: 10.3389/fendo.2022.1119154. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36726474 Free PMC article.
-
Opioid-induced adrenal insufficiency: diagnostic and management considerations.Front Endocrinol (Lausanne). 2024 Feb 27;14:1280603. doi: 10.3389/fendo.2023.1280603. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38476510 Free PMC article. Review.
References
-
- GBD 2017 Causes of Death Collaborators . Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet (London England) (2018) 392(10159):1736–88. doi: 10.1016/S0140-6736(18)32203-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

