Intratracheal administration of mesenchymal stem cells modulates lung macrophage polarization and exerts anti-asthmatic effects
- PMID: 35821386
- PMCID: PMC9276742
- DOI: 10.1038/s41598-022-14846-y
Intratracheal administration of mesenchymal stem cells modulates lung macrophage polarization and exerts anti-asthmatic effects
Abstract
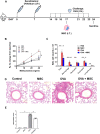
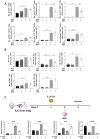
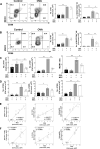
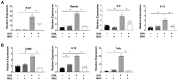
Mesenchymal stem cells (MSCs) possess immunomodulatory properties that have therapeutic potential for the treatment of inflammatory diseases. This study investigates the effects of direct MSC administration on asthmatic airways. Umbilical cord MSCs (ucMSCs) were intratracheally administered to six-week-old female BALB/c mice sensitized and challenged with ovalbumin; airway hyperresponsiveness (AHR), analyses of airway inflammatory cells, lung histology, flow cytometry, and quantitative real-time PCR were performed. Furthermore, ex vivo and in vitro experiments were performed to assess the effects of ucMSC on M2 activation. Intratracheally administered ucMSCs decreased degree of airway resistance and the number of inflammatory cells such as T helper 2 (Th2) cells, type 2 innate lymphoid cells (ILC2), and macrophages in the murine asthma model. Particularly, MHCII and CD86 expression diminished in dendritic cells and alveolar macrophages (AMs) following ucMSC treatment. SiglecF+CD11c+CD11b- AMs show a negative correlation with type II inflammatory cells including Th2 cells, ILC2, and eosinophils in asthmatic mice and were restored following intratracheal ucMSCs treatment. In addition, ucMSCs decreased the macrophage polarization to M2, particularly M2a. The expression levels of markers associated with M2 polarization and Th2 inflammation were also decreased. ucMSC reduced Il-12 and Tnfa expression as well as that of M2 markers such as Cd206 and Retnla ex vivo. Furthermore, the in vitro study using IL-4 treated macrophages confirmed that both direct and indirect MSC treatment significantly reduced the expression of Il-5 and Il-13. In conclusion, ucMSCs appear to suppress type II inflammation by regulating lung macrophages via soluble mediators.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Effect of Acinetobacter lwoffii on the modulation of macrophage activation and asthmatic inflammation.Clin Exp Allergy. 2022 Apr;52(4):518-529. doi: 10.1111/cea.14077. Epub 2021 Dec 16. Clin Exp Allergy. 2022. PMID: 34874580
-
Mesenchymal stem cells exert their anti-asthmatic effects through macrophage modulation in a murine chronic asthma model.Sci Rep. 2022 Jun 13;12(1):9811. doi: 10.1038/s41598-022-14027-x. Sci Rep. 2022. PMID: 35697721 Free PMC article.
-
Intravenous Mesenchymal Stem Cell Administration Modulates Monocytes/Macrophages and Ameliorates Asthmatic Airway Inflammation in a Murine Asthma Model.Mol Cells. 2022 Nov 30;45(11):833-845. doi: 10.14348/molcells.2022.0038. Epub 2022 Nov 11. Mol Cells. 2022. PMID: 36380733 Free PMC article.
-
Mesenchymal Stem Cells Attenuate Asthmatic Inflammation and Airway Remodeling by Modulating Macrophages/Monocytes in the IL-13-Overexpressing Mouse Model.Immune Netw. 2022 Sep 27;22(5):e40. doi: 10.4110/in.2022.22.e40. eCollection 2022 Oct. Immune Netw. 2022. PMID: 36381962 Free PMC article.
-
Key Role of Mesenchymal Stromal Cell Interaction with Macrophages in Promoting Repair of Lung Injury.Int J Mol Sci. 2023 Feb 8;24(4):3376. doi: 10.3390/ijms24043376. Int J Mol Sci. 2023. PMID: 36834784 Free PMC article. Review.
Cited by
-
From mesenchymal stem cells to their extracellular vesicles: Progress and prospects for asthma therapy.Asian J Pharm Sci. 2024 Aug;19(4):100942. doi: 10.1016/j.ajps.2024.100942. Epub 2024 Jul 9. Asian J Pharm Sci. 2024. PMID: 39253613 Free PMC article. Review.
-
Umbilical cord mesenchymal stem cells inhibited inflammation of bronchial epithelial cells by regulating Hedgehog pathway.Eur J Histochem. 2023 Dec 12;67(4):3908. doi: 10.4081/ejh.2023.3908. Eur J Histochem. 2023. PMID: 38085254 Free PMC article.
-
3D bioprinted mesenchymal stromal cells in skin wound repair.Front Surg. 2022 Oct 14;9:988843. doi: 10.3389/fsurg.2022.988843. eCollection 2022. Front Surg. 2022. PMID: 36311952 Free PMC article. Review.
-
ISX-9 Promotes KGF Secretion From MSCs to Alleviate ALI Through NGFR-ERK-TAU-β-Catenin Signaling Axis.Stem Cells Transl Med. 2024 Mar 15;13(3):255-267. doi: 10.1093/stcltm/szad085. Stem Cells Transl Med. 2024. PMID: 38159248 Free PMC article.
-
Mesenchymal Stem/Stromal Cells in Asthma Therapy: Mechanisms and Strategies for Enhancement.Cell Transplant. 2023 Jan-Dec;32:9636897231180128. doi: 10.1177/09636897231180128. Cell Transplant. 2023. PMID: 37318186 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

