ILP-2: A New Bane and Therapeutic Target for Human Cancers
- PMID: 35814477
- PMCID: PMC9260022
- DOI: 10.3389/fonc.2022.922596
ILP-2: A New Bane and Therapeutic Target for Human Cancers
Abstract
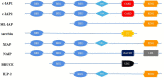
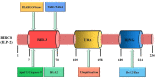
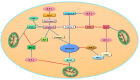
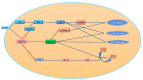
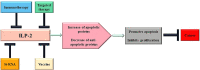
Inhibitor of apoptosis protein-related-like protein-2 (ILP-2), also known as BIRC-8, is a member of the inhibitor of apoptosis protein (IAPs) family, which mainly encodes the negative regulator of apoptosis. It is selectively overexpressed in a variety of human tumors and can help tumor cells evade apoptosis, promote tumor cell growth, increase tumor cell aggressiveness, and appears to be involved in tumor cell resistance to chemotherapeutic drugs. Several studies have shown that downregulation of ILP-2 expression increases apoptosis, inhibits metastasis, reduces cell growth potential, and sensitizes tumor cells to chemotherapeutic drugs. In addition, ILP-2 inhibits apoptosis in a unique manner; it does not directly inhibit the activity of caspases but induces apoptosis by cooperating with other apoptosis-related proteins. Here, we review the current understanding of the various roles of ILP-2 in the apoptotic cascade and explore the use of interfering ILP-2, and the combination of related anti-tumor agents, as a novel strategy for cancer therapy.
Keywords: IAPS; ILP-2; apoptosis; caspases; migration; tumor therapy.
Copyright © 2022 Zhang, Xiang, Cui, Peng, Mridul and Xiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Molecular cloning of ILP-2, a novel member of the inhibitor of apoptosis protein family.Mol Cell Biol. 2001 Jul;21(13):4292-301. doi: 10.1128/MCB.21.13.4292-4301.2001. Mol Cell Biol. 2001. PMID: 11390657 Free PMC article.
-
Fumonisin B₁ inhibits apoptosis in HepG2 cells by inducing Birc-8/ILP-2.Toxicol Lett. 2015 Jun 1;235(2):67-74. doi: 10.1016/j.toxlet.2015.03.006. Epub 2015 Mar 20. Toxicol Lett. 2015. PMID: 25800559
-
ILP-2 modeling and virtual screening of an FDA-approved library:a possible anticancer therapy.Turk J Med Sci. 2016 Jun 23;46(4):1135-43. doi: 10.3906/sag-1503-2. Turk J Med Sci. 2016. PMID: 27513416
-
Role of genomics-based strategies in overcoming chemotherapeutic resistance.Curr Pharm Biotechnol. 2004 Oct;5(5):471-80. doi: 10.2174/1389201043376698. Curr Pharm Biotechnol. 2004. PMID: 15544495 Review.
-
Challenge and promise: roles for Livin in progression and therapy of cancer.Mol Cancer Ther. 2008 Dec;7(12):3661-9. doi: 10.1158/1535-7163.MCT-08-0480. Mol Cancer Ther. 2008. PMID: 19074843 Review.
References
Publication types
LinkOut - more resources
Full Text Sources

