Selective Serotonin Reuptake Inhibitor-Treatment Does Not Show Beneficial Effects on Cognition or Amyloid Burden in Cognitively Impaired and Cognitively Normal Subjects
- PMID: 35813957
- PMCID: PMC9260503
- DOI: 10.3389/fnagi.2022.883256
Selective Serotonin Reuptake Inhibitor-Treatment Does Not Show Beneficial Effects on Cognition or Amyloid Burden in Cognitively Impaired and Cognitively Normal Subjects
Abstract
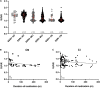
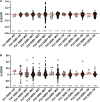
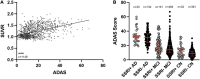
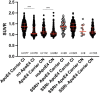
Preclinical studies indicate that selective serotonin reuptake inhibitors (SSRI) have beneficial effects on Alzheimer-related pathologies. Therefore, the aim of this study was to evaluate the influence of SSRI-treatment on amyloid burden in 18F-Florbetapir-positron emission tomography (PET) and on cognition in cognitively normal and cognitively impaired subjects. We included n = 755 cognitively impaired and n = 394 cognitively normal participants from the Alzheimer's Disease Neuroimaging Initiative (ADNI) that underwent at least one 18F-Florbetapir-PET. Standardized uptake ratios (SUVR) and the Alzheimer Disease Assessment Scale-cognitive subscale (ADAS) scores as well as follow-up results were compared between subgroups with a history of SSRI-treatment (SSRI+) and without SSRI-treatment (SSRI-) as well as in subgroups of SSRI+/Depression+ and SSRI+/Depression- and SSRI-/Depression+ and SSRI-/Depression-. 18F-Florbetapir-PET did not show significant differences of SUVR between the SSRI+ and SSRI- groups in both, cognitively impaired and cognitively normal participants. There were no differences in subgroups of SSRI+/Depression+ and SSRI+/Depression- and SSRI-/Depression+ and SSRI-/Depression-. However, SUVR showed a dose-dependent inverse correlation to the duration of medication in cognitively normal and in cognitively impaired patients. SRRI-treatment did not show an effect on ADAS scores. Furthermore, there was no effect on follow-up SUVR or on follow-up ADAS scores. Overall, SSRI-treatment did not show beneficial effects on amyloid load nor on cognition.
Keywords: Alzheheimer’s disease; amyloid-PET imaging; florbetaben; positron emission tomography; selective serotonin reuptake inhibitors.
Copyright © 2022 Bouter and Bouter.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Serotonin Selective Reuptake Inhibitor Treatment Improves Cognition and Grey Matter Atrophy but not Amyloid Burden During Two-Year Follow-Up in Mild Cognitive Impairment and Alzheimer's Disease Patients with Depressive Symptoms.J Alzheimers Dis. 2018;65(3):793-806. doi: 10.3233/JAD-170387. J Alzheimers Dis. 2018. PMID: 30010116
-
Cognition and amyloid load in Alzheimer disease imaged with florbetapir F 18(AV-45) positron emission tomography.Am J Geriatr Psychiatry. 2013 Mar;21(3):272-8. doi: 10.1016/j.jagp.2012.11.016. Am J Geriatr Psychiatry. 2013. PMID: 23395194 Free PMC article.
-
Amyloid-β assessed by florbetapir F 18 PET and 18-month cognitive decline: a multicenter study.Neurology. 2012 Oct 16;79(16):1636-44. doi: 10.1212/WNL.0b013e3182661f74. Epub 2012 Jul 11. Neurology. 2012. PMID: 22786606 Free PMC article.
-
Tracer-specific reference tissues selection improves detection of 18 F-FDG, 18 F-florbetapir, and 18 F-flortaucipir PET SUVR changes in Alzheimer's disease.Hum Brain Mapp. 2022 May;43(7):2121-2133. doi: 10.1002/hbm.25774. Epub 2022 Feb 15. Hum Brain Mapp. 2022. PMID: 35165964 Free PMC article.
-
18F PET with florbetapir for the early diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Nov 22;11(11):CD012216. doi: 10.1002/14651858.CD012216.pub2. Cochrane Database Syst Rev. 2017. PMID: 29164603 Free PMC article. Review.
Cited by
-
Anxiety and Alzheimer's disease pathogenesis: focus on 5-HT and CRF systems in 3xTg-AD and TgF344-AD animal models.Front Aging Neurosci. 2023 Nov 10;15:1251075. doi: 10.3389/fnagi.2023.1251075. eCollection 2023. Front Aging Neurosci. 2023. PMID: 38076543 Free PMC article. Review.
-
Association of amyloid-beta with depression or depressive symptoms in older adults without dementia: a systematic review and meta-analysis.Transl Psychiatry. 2024 Jan 15;14(1):25. doi: 10.1038/s41398-024-02739-9. Transl Psychiatry. 2024. PMID: 38225253 Free PMC article.
-
A multicenter, randomized, double-blind, placebo-controlled ascending dose study to evaluate the safety, tolerability, pharmacokinetics (PK) and pharmacodynamic (PD) effects of Posiphen in subjects with early Alzheimer's Disease.Alzheimers Res Ther. 2024 Jul 5;16(1):151. doi: 10.1186/s13195-024-01490-z. Alzheimers Res Ther. 2024. PMID: 38970127 Free PMC article. Clinical Trial.
-
A multicenter, randomized, double-blind, placebo-controlled ascending dose study to evaluate the safety, tolerability, pharmacokinetics (PK) and pharmacodynamic (PD) effects of Posiphen in subjects with Early Alzheimer's Disease.medRxiv [Preprint]. 2024 Mar 22:2024.03.20.24304638. doi: 10.1101/2024.03.20.24304638. medRxiv. 2024. Update in: Alzheimers Res Ther. 2024 Jul 5;16(1):151. doi: 10.1186/s13195-024-01490-z. PMID: 38562783 Free PMC article. Updated. Preprint.
-
The Alzheimer's Disease Neuroimaging Initiative in the era of Alzheimer's disease treatment: A review of ADNI studies from 2021 to 2022.Alzheimers Dement. 2024 Jan;20(1):652-694. doi: 10.1002/alz.13449. Epub 2023 Sep 12. Alzheimers Dement. 2024. PMID: 37698424 Free PMC article. Review.
References
-
- Bartels C., Belz M., Vogelgsang J., Hessmann P., Bohlken J., Wiltfang J., et al. (2020). To Be Continued? Long-Term Treatment Effects of Antidepressant Drug Classes and Individual Antidepressants on the Risk of Developing Dementia: a German Case-Control Study. J. Clin. Psychiatry 81:19m13205. 10.4088/JCP.19m13205 - DOI - PubMed
-
- Bartels C., Wagner M., Wolfsgruber S., Ehrenreich H., Schneider A., Alzheimer’s Disease Neuroimaging I. (2018). Impact of SSRI Therapy on Risk of Conversion From Mild Cognitive Impairment to Alzheimer’s Dementia in Individuals With Previous Depression. Am. J. Psychiatry 175 232–241. 10.1176/appi.ajp.2017.17040404 - DOI - PubMed
-
- Barthel H., Sabri O. (2017). Clinical Use and Utility of Amyloid Imaging. J. Nucl. Med. 58 1711–1717. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

