Cytomegalovirus Management in Solid Organ Transplant Recipients: A Pre-COVID-19 Survey From the Working Group of the European Society for Organ Transplantation
- PMID: 35812158
- PMCID: PMC9257585
- DOI: 10.3389/ti.2022.10332
Cytomegalovirus Management in Solid Organ Transplant Recipients: A Pre-COVID-19 Survey From the Working Group of the European Society for Organ Transplantation
Abstract
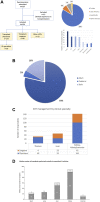
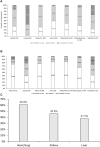
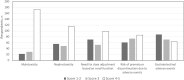
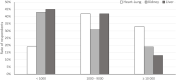
Infections are leading causes of morbidity/mortality following solid organ transplantation (SOT) and cytomegalovirus (CMV) is among the most frequent pathogens, causing a considerable threat to SOT recipients. A survey was conducted 19 July-31 October 2019 to capture clinical practices about CMV in SOT recipients (e.g., how practices aligned with guidelines, how adequately treatments met patients' needs, and respondents' expectations for future developments). Transplant professionals completed a ∼30-minute online questionnaire: 224 responses were included, representing 160 hospitals and 197 SOT programs (41 countries; 167[83%] European programs). Findings revealed a heterogenous approach to CMV diagnosis and management and, sometimes, significant divergence from international guidelines. Valganciclovir prophylaxis (of variable duration) was administered by 201/224 (90%) respondents in D+/R- SOT and by 40% in R+ cases, with pre-emptive strategies generally reserved for R+ cases: DNA thresholds to initiate treatment ranged across 10-10,000 copies/ml. Ganciclovir-resistant CMV strains were still perceived as major challenges, and tailored treatment was one of the most important unmet needs for CMV management. These findings may help to design studies to evaluate safety and efficacy of new strategies to prevent CMV disease in SOT recipients, and target specific educational activities to harmonize CMV management in this challenging population.
Keywords: ESOT; cellular immunity; infection cytomegalovirus; organ transplantation; pre-emptive therapy; prophylaxis; survey.
Copyright © 2022 Grossi, Kamar, Saliba, Baldanti, Aguado, Gottlieb, Banas and Potena.
Conflict of interest statement
PG has been a consultant or member of an advisory committee for Angelini, Becton Dickinson, Biotest, Correvio, Gilead Sciences, Merck Sharpe & Dohme, Nordic Pharma, Paratek Pharmaceuticals, AlloVir, and Shire; and a speakers’ bureau member for Becton Dickinson, Biotest, Gilead Sciences, Merck Sharpe & Dohme, Pfizer, Atara, and Vertex. NK has been a speaker or an advisory board member for AbbVie, Amgen, Astellas, Biotest, Chiesi, CSL Behring, Gilead, Fresenius Medical Care, Merck Sharpe & Dohme, Neovii, Novartis Pharma, Sandoz, Sanofi, and Shire. FS has received speaker’s honoraria and/or research grants from Novartis, Astellas, Chiesi, Gilead, Merck Sharp & Dohme, Neovii, Sandoz, Pfizer, Biotest, Takeda, and Baxter. FB has received grants/research support from AB Analitica, DiaSorin, ELITechGroup, NTP, and Qiagen; he has also received honoraria or consultation fees from Biotest, DiaSorin, HUMABS, Merck Sharpe & Dohme, Qiagen, and Shire. JA has been a consultant and speakers’ bureau member for Astellas Pharma, Biotoscana, Gilead, Merck Sharpe & Dohme, Pfizer, Roche, and United Medical. LP has been a consultant for Novartis and Biotest and received speaker fees from AstraZeneca, Boehringer Ingelheim, Abbott, and Paragonix. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
New developments in the management of cytomegalovirus infection after solid organ transplantation.Drugs. 2010 May 28;70(8):965-81. doi: 10.2165/10898540-000000000-00000. Drugs. 2010. PMID: 20481654 Review.
-
Ganciclovir-resistant cytomegalovirus infection in solid organ transplant recipients: a single-center retrospective cohort study.Transpl Infect Dis. 2016 Jun;18(3):390-5. doi: 10.1111/tid.12537. Epub 2016 Jun 9. Transpl Infect Dis. 2016. PMID: 27037651
-
Cytomegalovirus infection management in solid organ transplant recipients across European centers in the time of molecular diagnostics: An ESGICH survey.Transpl Infect Dis. 2017 Dec;19(6). doi: 10.1111/tid.12773. Epub 2017 Oct 25. Transpl Infect Dis. 2017. PMID: 28859257
-
The next frontier: cytomegalovirus antiviral stewardship programs in solid organ transplant.Curr Opin Infect Dis. 2023 Dec 1;36(6):497-504. doi: 10.1097/QCO.0000000000000963. Epub 2023 Oct 10. Curr Opin Infect Dis. 2023. PMID: 37815319 Review.
-
Cytomegalovirus in Solid Organ Transplant Recipients: Clinical Updates, Challenges and Future Directions.Curr Pharm Des. 2020;26(28):3497-3506. doi: 10.2174/1381612826666200531152901. Curr Pharm Des. 2020. PMID: 32473617 Review.
Cited by
-
Innate and adaptive effector immune drivers of cytomegalovirus disease in lung transplantation: a double-edged sword.Front Transplant. 2024 Jun 10;3:1388393. doi: 10.3389/frtra.2024.1388393. eCollection 2024. Front Transplant. 2024. PMID: 38993763 Free PMC article. Review.
-
Diagnosis of Human Cytomegalovirus Drug Resistance Mutations in Solid Organ Transplant Recipients-A Review.Diagnostics (Basel). 2024 Jan 18;14(2):203. doi: 10.3390/diagnostics14020203. Diagnostics (Basel). 2024. PMID: 38248079 Free PMC article. Review.
-
Understanding the Cytomegalovirus Cyclin-Dependent Kinase Ortholog pUL97 as a Multifaceted Regulator and an Antiviral Drug Target.Cells. 2024 Aug 13;13(16):1338. doi: 10.3390/cells13161338. Cells. 2024. PMID: 39195228 Free PMC article. Review.
-
Management of cytomegalovirus infection after liver transplantation.World J Transplant. 2024 Sep 18;14(3):93209. doi: 10.5500/wjt.v14.i3.93209. World J Transplant. 2024. PMID: 39295968 Free PMC article. Review.
-
A practical guide to real-world implementation of pre-emptive therapy for Cytomegalovirus disease prevention in high-risk seronegative liver transplant recipients with seropositive donors.Transpl Infect Dis. 2024 Jun;26(3):e14229. doi: 10.1111/tid.14229. Epub 2024 Jan 12. Transpl Infect Dis. 2024. PMID: 38214192
References
-
- Hakimi N, Dorey J, Hakimi Z, Aballea S, Odeyemi II, Toumi M. PIN77 Elicitation of Health-Related Quality of Life Concepts Associated with Cytomegalovirus in Transplant Recipients. Value in Health (2012) 15:A399. 10.1016/j.jval.2012.08.1144 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

