N-(2-Arylethyl)-2-methylprop-2-enamides as Versatile Reagents for Synthesis of Molecularly Imprinted Polymers
- PMID: 35808783
- PMCID: PMC9269059
- DOI: 10.3390/polym14132738
N-(2-Arylethyl)-2-methylprop-2-enamides as Versatile Reagents for Synthesis of Molecularly Imprinted Polymers
Abstract
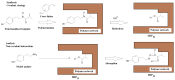
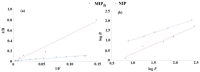
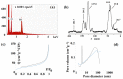
The paper describes the formation of six aromatic N-(2-arylethyl)-2-methylprop-2-enamides with various substituents in benzene ring, viz., 4-F, 4-Cl, 2,4-Cl2, 4-Br, 4-OMe, and 3,4-(OMe)2 from 2-arylethylamines and methacryloyl chloride in ethylene dichloride with high yields (46-94%). The structure of the compounds was confirmed by 1H NMR, 13C NMR, IR, and HR-MS. Those compounds were obtained to serve as functionalized templates for the fabrication of molecularly imprinted polymers followed by the hydrolysis of an amide linkage. In an exemplary experiment, the imprinted polymer was produced from N-(2-(4-bromophenyl)ethyl)-2-methylprop-2-enamide and divinylbenzene, acting as cross-linker. The hydrolysis of 2-(4-bromophenyl)ethyl residue proceeded and the characterization of material including SEM, EDS, 13C CP MAS NMR, and BET on various steps of preparation was carried out. The adsorption studies proved that there was a high affinity towards the target biomolecules tyramine and L-norepinephrine, with imprinting factors equal to 2.47 and 2.50, respectively, when compared to non-imprinted polymer synthesized from methacrylic acid and divinylbenzene only.
Keywords: N-acylation; molecularly imprinted polymers; phenethylamines; semi-covalent imprinting; tyramine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
A highly selective molecularly imprinted sorbent for extraction of 2-aminothiazoline-4-carboxylic acid--Synthesis, characterization and application in post-mortem whole blood analysis.J Chromatogr A. 2015 Nov 13;1420:16-25. doi: 10.1016/j.chroma.2015.09.083. Epub 2015 Oct 1. J Chromatogr A. 2015. PMID: 26463428
-
Preparation of melamine molecularly imprinted polymer by computer-aided design.J Sep Sci. 2015 Aug;38(15):2647-54. doi: 10.1002/jssc.201500375. Epub 2015 Jun 13. J Sep Sci. 2015. PMID: 25964122
-
Effect of functional monomers and porogens on morphology, structure and recognition properties of 2-(4-methoxyphenyl)ethylamine imprinted polymers.Mater Sci Eng C Mater Biol Appl. 2013 Apr 1;33(3):1162-9. doi: 10.1016/j.msec.2012.12.006. Epub 2012 Dec 11. Mater Sci Eng C Mater Biol Appl. 2013. PMID: 23827555
-
Molecularly imprinted polymer grafted on paper and flat sheet for selective sensing and diagnosis: a review.Mikrochim Acta. 2021 Jul 30;188(8):279. doi: 10.1007/s00604-021-04930-x. Mikrochim Acta. 2021. PMID: 34331135 Review.
-
Factors Affecting Preparation of Molecularly Imprinted Polymer and Methods on Finding Template-Monomer Interaction as the Key of Selective Properties of the Materials.Molecules. 2021 Sep 16;26(18):5612. doi: 10.3390/molecules26185612. Molecules. 2021. PMID: 34577083 Free PMC article. Review.
Cited by
-
Editorial: Advance in Molecularly Imprinted Polymers.Polymers (Basel). 2023 Jul 27;15(15):3199. doi: 10.3390/polym15153199. Polymers (Basel). 2023. PMID: 37571093 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

