Effect of Silver Nanoparticles on the In Vitro Regeneration, Biochemical, Genetic, and Phenotype Variation in Adventitious Shoots Produced from Leaf Explants in Chrysanthemum
- PMID: 35806413
- PMCID: PMC9266331
- DOI: 10.3390/ijms23137406
Effect of Silver Nanoparticles on the In Vitro Regeneration, Biochemical, Genetic, and Phenotype Variation in Adventitious Shoots Produced from Leaf Explants in Chrysanthemum
Abstract
Novel and unique properties of nanomaterials, which are not apparent in larger-size forms of the same material, encourage the undertaking of studies exploring the multifaced effects of nanomaterials on plants. The results of such studies are not only scientifically relevant but, additionally, can be implemented to plant production and/or breeding. This study aimed to verify the applicability of silver nanoparticles (AgNPs) as a mutagen in chrysanthemum breeding. Chrysanthemum × grandiflorum (Ramat.) Kitam. 'Lilac Wonder' and 'Richmond' leaf explants were cultured on the modified MS medium supplemented with 0.6 mg·L-1 6-benzylaminopurine (BAP) and 2 mg·L-1 indole-3-acetic acid (IAA) and treated with AgNPs (spherical; 20 nm in diameter size; 0, 50, and 100 mg·L-1). AgNPs strongly suppressed the capability of leaf explants to form adventitious shoots and the efficiency of shoot regeneration. The content of primary and secondary metabolites (chlorophyll a, chlorophyll b, total chlorophylls, carotenoids, anthocyanins, phenolic compounds) and the activity of enzymatic antioxidants (superoxide dismutase and guaiacol peroxide) in leaf explants varied depending on the AgNPs treatment and age of culture. Phenotype variations of ex vitro cultivated chrysanthemums, covering the color and pigment content in the inflorescence, were detected in one 50 mg·L-1 AgNPs-derived and five 100 mg·L-1 AgNPs-derived 'Lilac Wonder' plants and were manifested as the color change from pink to burgundy-gold. However, no changes in inflorescence color/shape were found among AgNPs-treated 'Richmond' chrysanthemums. On the other hand, the stem height, number of leaves, and chlorophyll content in leaves varied depending on the AgNPs treatment and the cultivar analyzed. A significant effect of AgNPs on the genetic variation occurrence was found. A nearly two-fold higher share of polymorphic products, in both cultivars studied, was generated by RAPD markers than by SCoTs. To conclude, protocols using leaf explant treatment with AgNPs can be used as a novel breeding technique in chrysanthemum. However, the individual cultivars may differ in biochemical response, the efficiency of in vitro regeneration, genetic variation, and frequency of induced mutations in flowering plants.
Keywords: Chrysanthemum × grandiflorum (Ramat.) Kitam.; induced mutagenesis; molecular markers; nanotechnology; oxidative stress; phenotype alternation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



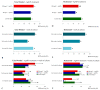
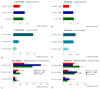

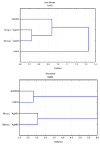


Similar articles
-
Synthesis, Characteristics, and Effect of Zinc Oxide and Silver Nanoparticles on the In Vitro Regeneration and Biochemical Profile of Chrysanthemum Adventitious Shoots.Materials (Basel). 2022 Nov 18;15(22):8192. doi: 10.3390/ma15228192. Materials (Basel). 2022. PMID: 36431675 Free PMC article.
-
Effect of nanoparticles on the ex-vitro performance of cryopreservation-derived plant material.PLoS One. 2024 Sep 12;19(9):e0310424. doi: 10.1371/journal.pone.0310424. eCollection 2024. PLoS One. 2024. PMID: 39264924 Free PMC article.
-
Biosynthesis and characterization of silver nanoparticles using Ochradenus arabicus and their physiological effect on Maerua oblongifolia raised in vitro.Sci Rep. 2020 Oct 16;10(1):17569. doi: 10.1038/s41598-020-74675-9. Sci Rep. 2020. PMID: 33067571 Free PMC article.
-
Molecular mechanisms underlying the diverse array of petal colors in chrysanthemum flowers.Breed Sci. 2018 Jan;68(1):119-127. doi: 10.1270/jsbbs.17075. Epub 2018 Feb 17. Breed Sci. 2018. PMID: 29681754 Free PMC article. Review.
-
Towards the Improvement of Ornamental Attributes in Chrysanthemum: Recent Progress in Biotechnological Advances.Int J Mol Sci. 2022 Oct 14;23(20):12284. doi: 10.3390/ijms232012284. Int J Mol Sci. 2022. PMID: 36293140 Free PMC article. Review.
Cited by
-
Biosynthesis of Silver Nanoparticles and Exploring Their Potential of Reducing the Contamination of the In Vitro Culture Media and Inducing the Callus Growth of Rumex nervosus Explants.Molecules. 2023 Apr 23;28(9):3666. doi: 10.3390/molecules28093666. Molecules. 2023. PMID: 37175076 Free PMC article.
-
Synthesis, Characteristics, and Effect of Zinc Oxide and Silver Nanoparticles on the In Vitro Regeneration and Biochemical Profile of Chrysanthemum Adventitious Shoots.Materials (Basel). 2022 Nov 18;15(22):8192. doi: 10.3390/ma15228192. Materials (Basel). 2022. PMID: 36431675 Free PMC article.
-
Effect of nanoparticles on the ex-vitro performance of cryopreservation-derived plant material.PLoS One. 2024 Sep 12;19(9):e0310424. doi: 10.1371/journal.pone.0310424. eCollection 2024. PLoS One. 2024. PMID: 39264924 Free PMC article.
References
-
- Yadollahi A., Arzani K., Khoshghalb H. The role of nanotechnology in horticultural crops postharvest management. Acta Hortic. 2010;875:49–56. doi: 10.17660/ActaHortic.2010.875.4. - DOI
-
- Milewska-Hendel A., Gawecki R., Zubko M., Stróż D., Kurczyńska E. Diverse influence of nanoparticles on plant growth with a particular emphasis on crop plants. Acta Agrobot. 2016;69:1694. doi: 10.5586/aa.1694. - DOI
-
- Fayez K.A., El-Deeb B.A., Mostafa N.Y. Toxicity of biosynthetic silver nanoparticles on the growth, cell ultrastructure and physiological activities of barley plant. Acta Physiol. Plant. 2017;39:155. doi: 10.1007/s11738-017-2452-3. - DOI
-
- Singh A., Singh N.B., Afzal S., Singh T., Hussain I. Zinc oxide nanoparticles: A review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J. Mater. Sci. 2018;53:185–201. doi: 10.1007/s10853-017-1544-1. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

