Erythropoietin in Optic Neuropathies: Current Future Strategies for Optic Nerve Protection and Repair
- PMID: 35806148
- PMCID: PMC9267007
- DOI: 10.3390/ijms23137143
Erythropoietin in Optic Neuropathies: Current Future Strategies for Optic Nerve Protection and Repair
Abstract
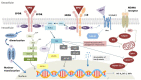
Erythropoietin (EPO) is known as a hormone for erythropoiesis in response to anemia and hypoxia. However, the effect of EPO is not only limited to hematopoietic tissue. Several studies have highlighted the neuroprotective function of EPO in extra-hematopoietic tissues, especially the retina. EPO could interact with its heterodimer receptor (EPOR/βcR) to exert its anti-apoptosis, anti-inflammation and anti-oxidation effects in preventing retinal ganglion cells death through different intracellular signaling pathways. In this review, we summarized the available pre-clinical studies of EPO in treating glaucomatous optic neuropathy, optic neuritis, non-arteritic anterior ischemic optic neuropathy and traumatic optic neuropathy. In addition, we explore the future strategies of EPO for optic nerve protection and repair, including advances in EPO derivates, and EPO deliveries. These strategies will lead to a new chapter in the treatment of optic neuropathy.
Keywords: erythropoietin; neuroprotection; optic nerve protection; optic neuropathy; retinal ganglion cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The Latest Evidence of Erythropoietin in the Treatment of Glaucoma.Int J Mol Sci. 2022 Dec 16;23(24):16038. doi: 10.3390/ijms232416038. Int J Mol Sci. 2022. PMID: 36555679 Free PMC article. Review.
-
Up-regulated endogenous erythropoietin/erythropoietin receptor system and exogenous erythropoietin rescue retinal ganglion cells after chronic ocular hypertension.Cell Mol Neurobiol. 2008 Feb;28(2):317-29. doi: 10.1007/s10571-007-9155-z. Epub 2007 Jun 7. Cell Mol Neurobiol. 2008. PMID: 17554621
-
[Erythropoietin protects retinal ganglion cells and visual function after ocular ischemia and optic nerve compression].Ophthalmologe. 2010 Apr;107(4):347-53. doi: 10.1007/s00347-009-2030-1. Ophthalmologe. 2010. PMID: 19787355 German.
-
Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor.Proc Natl Acad Sci U S A. 2004 Oct 12;101(41):14907-12. doi: 10.1073/pnas.0406491101. Epub 2004 Sep 29. Proc Natl Acad Sci U S A. 2004. PMID: 15456912 Free PMC article.
-
Erythropoietin, erythropoiesis and beyond.Biochem Pharmacol. 2011 Nov 15;82(10):1291-303. doi: 10.1016/j.bcp.2011.06.045. Epub 2011 Jul 7. Biochem Pharmacol. 2011. PMID: 21782802 Review.
Cited by
-
Serum Erythropoietin and Ischemic-Modified Albumin Levels in Adolescents with Obsessive-Compulsive Disorder.J Mol Neurosci. 2024 Jul 12;74(3):67. doi: 10.1007/s12031-024-02247-x. J Mol Neurosci. 2024. PMID: 38995319 Free PMC article.
-
Erythropoietin Protects against Retinal Damage in A Rat Model of Optic Neuropathy via Glial Suppression.Cell J. 2023 May 28;25(5):327-337. doi: 10.22074/cellj.2023.1971689.1159. Cell J. 2023. PMID: 37300294 Free PMC article.
-
Optic Neuropathies: Current and Future Strategies for Optic Nerve Protection and Repair.Int J Mol Sci. 2023 Apr 10;24(8):6977. doi: 10.3390/ijms24086977. Int J Mol Sci. 2023. PMID: 37108140 Free PMC article.
-
Commentary: Erythropoietin as a treatment option for indirect optic neuropathy: A pilot study.Indian J Ophthalmol. 2023 Jan;71(1):241. doi: 10.4103/ijo.IJO_2248_22. Indian J Ophthalmol. 2023. PMID: 36588243 Free PMC article. No abstract available.
-
The role of the mTOR pathway in diabetic retinopathy.Front Med (Lausanne). 2022 Nov 1;9:973856. doi: 10.3389/fmed.2022.973856. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36388931 Free PMC article. Review.
References
-
- Yasuoka Y., Fukuyama T., Izumi Y., Nakayama Y., Inoue H., Yanagita K., Oshima T., Yamazaki T., Uematsu T., Kobayashi N., et al. Erythropoietin production by the kidney and the liver in response to severe hypoxia evaluated by Western blotting with deglycosylation. Physiol. Rep. 2020;8:e14485. doi: 10.14814/phy2.14485. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

